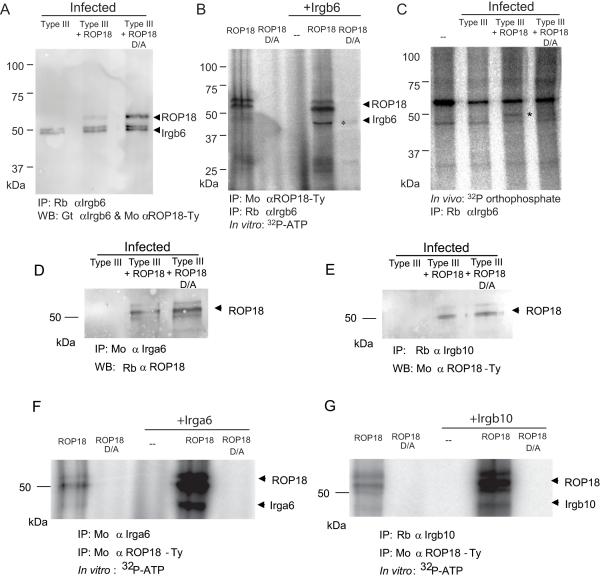
Figure 4. ROP18 coprecipitates and phosphorylates IRGs.
(A) Immunoprecipitation (IP) of Irgb6 from macrophages infected with type III, type III expressing active ROP18 (Type III + ROP18), or type III expressing the kinase-dead form of ROP18 (Type III +ROP18 D/A). IP with specific rabbit antiserum (Rb) against Irgb6; proteins were resolved by SDS-PAGE and Western blotted with goat (Gt) αIrgb6 and mouse (Mo) αTy to detect ROP18. Doublet of Irgb6 corresponds to an unknown modification.
(B) Phosphorylation of ROP18 and Irgb6 in a kinase reaction in vitro. Immunoprecipitated ROP18 (Mo αTy) and Irgb6 (Rb αIrgb6) were incubated with 32P-ATP in a kinase reaction, resolved by SDS-PAGE, and signals detected by phosphorimaging. * corresponds to Irgb6. Multiple autophorphorylation bands in ROP18 represent different processing forms as described previously (Qiu et al., 2008); the intensity of labeling characteristically changes in the presence of substrate. ROP18 migrates slightly faster in the presence of Irgb6; however, no other proteins were detected, nor modifications other than phosphorylation, were detected by MS analysis.
(C) In vivo phosphorylation of Irgb6 by ROP18. IFN-γ-activated macrophages were labeled with 32P orthophosphate, infected for 30 min, and IP with Rb αIrgb6. * corresponds to Irgb6 as verified by MS/MS identification. Additional bands reflect contaminating host kinase activity that is unrelated to ROP18.
(D, E) IP of Irga6 (D) or Irgb10 (E) from IFN-γ-activated macrophages with either mouse antiIrga6 (Mo αIrgb6) or rabbit anti-Irgb10 (Rb αIrgb10) at 1 h post infection. Proteins were resolved by SDS-PAGE and blotted with rabbit anti-ROP18 (Rb αROP18) or mAb to the Ty epitope tag (Mo α ROP18-Ty). Multiple bands in ROP18 represent processing as described previously (Qiu et al., 2008).
(F, G) Phosphorylation of Irga6 (F) or Irgb10 (G) by ROP18 in an in vitro kinase reaction. ROP18 was immunoprecipitated (IP: Mo αTy) from extracellular parasites expressing either active kinase (ROP18) or the inactive mutant (ROP18 D/A) and combined with immunoprecipitated Irga6 (IP: Mo αIrga6) or Irgb10 (IP: Rb αIrgb10) isolated from IFN-γ activated macrophages. Proteins were incubated with 32P-ATP in a kinase reaction, resolved by SDS-PAGE, and signals detected by phosphorimaging. Multiple bands in ROP18 represent processing as described previously (Qiu et al., 2008).