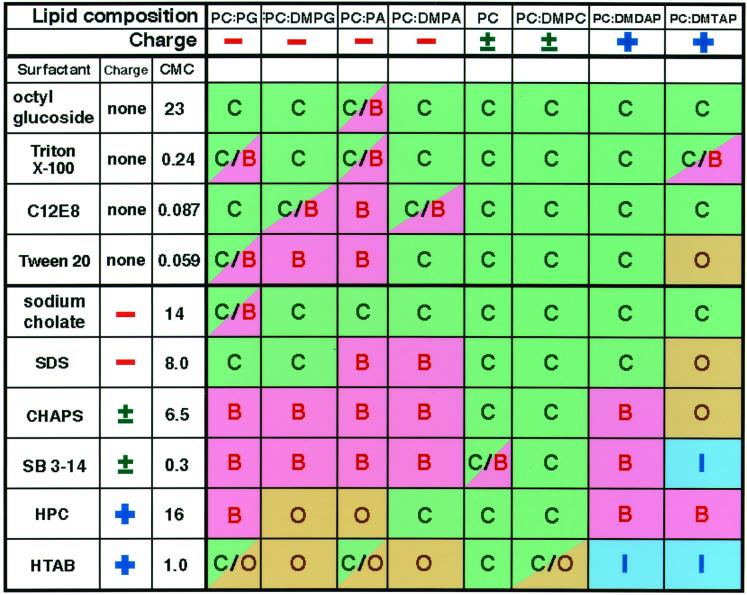
Figure 1.
Summary of the solubilization processes. C, continuous-stepwise or -smooth shrinkage; I, inside-out inversion; O, opening up; B, burst. Lipids: PC, phosphatidylcholine; DMPC, dimyristoyl phosphatidylcholine; PG, phosphatidylglycerol; DMPG, dimyristoyl phosphatidylglycerol; PA, phosphatidic acid; DMPA, dimyristoyl phosphatidic acid; DMDAP, 1,2-dimyristoyl 3-dimethylammonium propane; DMTAP, 1,2-dimyristoyl 3-trimethylammonium propane. Among eight kinds of lipids, PC, PG, and PA are isolated from egg yolk or other natural sources, so that they had nonuniform lengths of acyl chains, and others had uniform tail lengths (14 C). PC, DMPC, PG, and DMPG had large head groups, and PA, DMPA, DMDAP and DMTAP had small head groups. Among the eight kinds of liposomes used, seven had binary lipid compositions (1:1 mol/mol). The charge carried by liposomes at neutral pH is shown. The surfactants shown here were added to each liposome solution. Abbreviations for surfactants: C12E8, polyoxyethylene 8 lauryl ether; CHAPS, 3-[(3-cholamidopropyl) dimethylammonio]-1-propane sulfonate; SB 3–14, N-tetradecyl-N, N-dimethyl-3-ammonio-1-propane sulfonate; HPC, hexadecyl pyridinium chloride; HTAB, hexadecyl trimethyl ammonium bromide. The charge carried by each surfactant at neutral pH and its critical micelle concentration (mM) is shown. We usually selected giant liposomes whose diameters exceeded 5 μm for observations to make them and later analysis easy. The experimental results shown here were essentially not altered even when the concentration of surfactant was changed severalfold; however, the rate of liposomal solubilization in each process was changed. It is noted that, by monitoring the changing of solution turbidity, we confirmed that liposomal solubilization started when the concentrations of each added surfactant were higher than its critical micelle concentration.