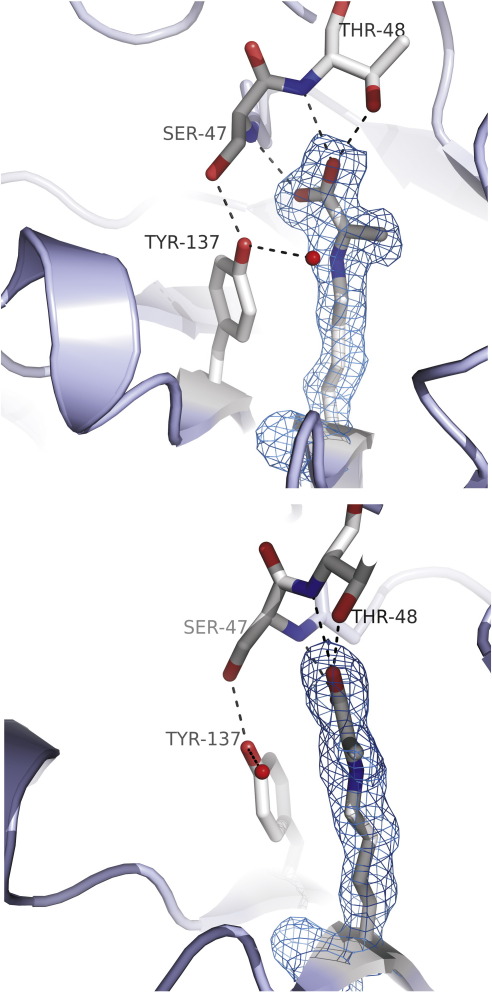
Fig. 5.
Active site of the wild-type NAL–pyruvate complex. Two views of the active site of the wild-type NAL–pyruvate complex showing the interactions of the pyruvate enamine with the enzyme. Pyruvate is covalently linked to Lys165 and makes hydrogen bonds with Ser47 and Thr48. The hydrogen-bonding network to Tyr137 and a conserved water molecule are also shown. The lower panel shows a rotation of the molecule to display the planar nature of the 2Fo − Fc electron density (contoured at 1 RMSD) of pyruvate–enzyme bonding confirming the enamine structure for this intermediate.