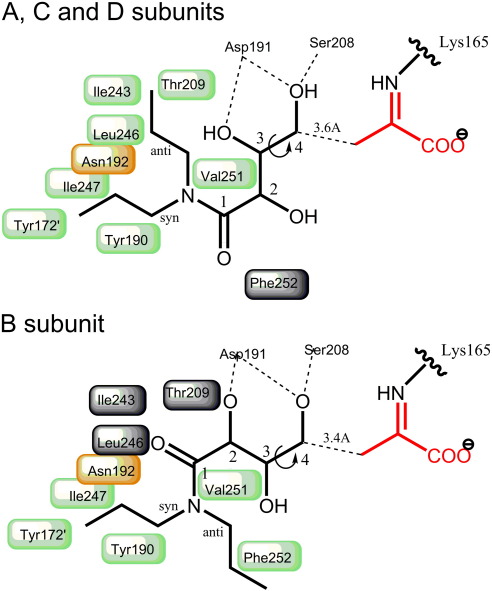
Fig. 8.
Schematic representation of THB binding to E192N NAL. Top: Binding of THB in subunits A, C, and D. THB (black lines) binds in the active-site pocket, with its C4 positioned 3.6 Å from the C3 of pyruvate (red) bound as a Schiff base to Lys165. Ser208 and Asp191 make H-bonds with the C4–OH and C3–OH groups. Asn192 (orange shaded box) lies between the syn-propyl arm and the anti-propyl arm. The hydrophobic sites (green shaded boxes) for these arms are formed from Tyr190, Ile247, and Tyr172′ (syn), and a deep pocket is formed by Leu246, Ile243, Thr209, and Val251 (anti). In this binding mode, Phe252 (gray shaded box) appears to have little binding role. Bottom: Binding of THB in subunit B. Rotation of THB around the C3–C4 bond compared with binding in subunits A, C, and D positions THB such that Ser208 and Asp191 now H-bond to the C4–OH and C2–OH groups and Asn192 lies between the syn arm and the C1 carbonyl group. This rotation positions the syn arm in the same hydrophobic binding site (green boxes); however, in this orientation, the anti arm is bound against Val251 and Phe252 (green boxes), and the deep hydrophobic pocket (gray boxes) is empty. Note that Val251 lies in a position where it can form part of the deep hydrophobic pocket and the site for the anti arm in subunit B.