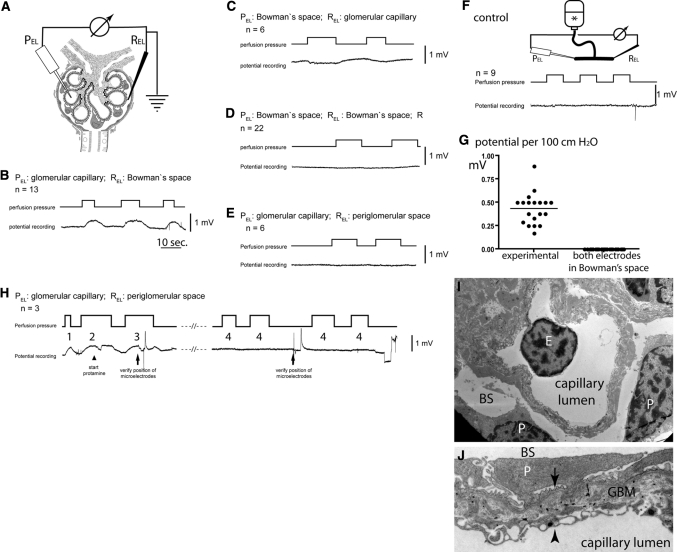
Figure 2.
Direct detection of a filtration-dependent potential difference in vivo. (A) Circuit diagram. The microelectrodes are positioned at defined anatomic locations within the renal glomerulus (i.e., corpuscle). (B–E) Representative potential measurements when perfusion pressures are changed at intervals as indicated. The anatomic location of the respective microelectrodes and the number of independent experiments (n) are indicated (PEL, potential microelectrode; REL, reference electrode). (F) Control experiment. To exclude artifacts from tip or flow potentials, the microelectrodes were placed into a communicating tube system filled with perfusion solution (*), and pressures were increased at intervals as indicated. As shown in a representative recording, no potential changes were observed. (G) Summary of the mean filtration-dependent potential difference in 19 glomeruli of 10 animals (as shown in B and C) and 22 control experiments (as shown in D). (H) Effect of protamine on the filtration-dependent potential difference. After micropuncture of a glomerulus, the correct position of the microelectrodes was verified by lissamine injection (data not shown) and detection of the filtration dependent potential difference (H1 and H2). Subsequently, protamine was added to the perfusate (arrowhead). A filtration dependent potential difference was detectable for up to 100 seconds with decreasing amplitude (H3). After >150 seconds, no filtration-dependent potential difference could be measured in this and at least two additional glomeruli (H4). (I) By transmission electron microscopy, the microanatomy of the Necturus glomerular filtration barrier was unaffected after perfusion with protamine (E, endothelial cell; P, podocyte; BS, Bowman's space). (J) Higher magnification of the Necturus filtration barrier (same animal as in H and I). The endothelial cells form cellular processes and multiple pores to allow filtration to occur (arrowhead). The space (glomerular basement membrane) between the podocyte foot processes (arrow) and endothelial cells is wider than in the mammalian glomerulus and filled with loose extracellular matrix, which also contains type 1 collagen (electron-dense fibers) (P, podocyte primary process; BS, Bowman's space).