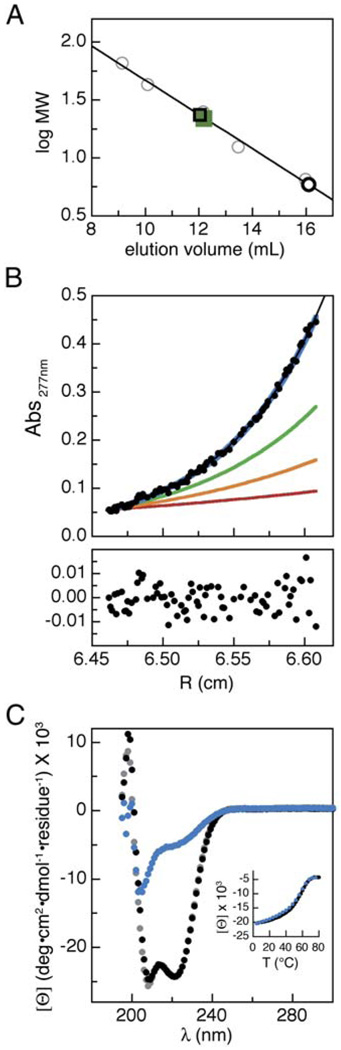
Figure 3. The TRPM8 Coiled-Coil Peptide Forms Tetramers.
(A) Gel filtration chromatography data for purified coiled-coil peptide (1055cc) (green squares). Standards (gray circles), predicted monomer (open black circle), and predicted tetramer (open black squares) elution volumes are indicated.
(B) 1055cc peptide analytical ultracentrifugation. Data are shown for 100 µM 1055cc at 27,000 rpm at Abs277 nm. Top panel shows the data (black points), single-species fit (black line), and curves for predicted monomeric (red), dimeric (orange), trimeric (green), and tetrameric (blue) species. Bottom panel shows residual errors of the single-species fit of the data.
(C) Circular dichroism spectra of 100 µM 1055cc peptide at 4°C (black), 80°C (blue), and 4°C post-melt (gray). Inset shows signal at 222 nm during the forward (black) and reverse (blue) temperature scans.