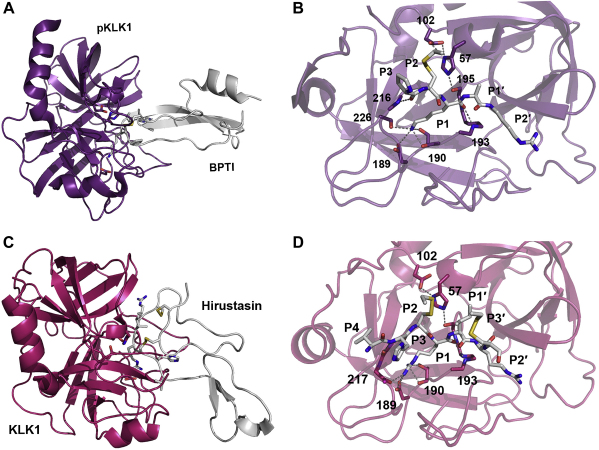
Fig. 8.
Two complexes of porcine and human KLK1 with the exogenous inhibitors BPTI (Kunitz-type) and hirustasin (antistasin-type), respectively. Proteins are shown as ribbon representation, relevant residues as sticks labelled with numbers, and hydrogen bonds as grey dotted lines. A: pKLK1-BPTI complex displayed with the protease in purple and the inhibitor in white. B: Closer view of the active pKLK1 site, where only the interacting reactive loop residues of BPTI are shown. The P1 Lys is bound with its carbonyl O to the oxyanion hole, while its Nζ-atom is hydrogen bonded to the Thr190 Oγ and carbonyl O, the Ser226 Oγ and virtually not to the Oδ1 of Asp189. The P2 Cys14I-Cys38I (I for inhibitor chain) disulfide bridge occupies the S2 pocket, while the P3 Pro only forms one hydrogen bond with its carbonyl O to the NH of Gly216. In contrast to many other canonical binding inhibitors, no interaction of the P4 residue Gly is observed. On the prime side, P1′ Ala fits to the S1, pocket, but the P2′ Arg exhibits a non-canonical binding around the S2, and S3, subsites. C: KLK1-hirustasin complex depicted with the protease in magenta and the inhibitor in white. D: Closer view of the active KLK1 site, where the inhibitor residues P4 to P3′ are displayed, although canonical binding ends with P1′. The P1 Arg of hirustasin is bound the oxyanion hole and its side chain fills the S1 pocket largely, whereby the Nη1 atom is hydrogen bonded to the Thr190 Oγ and carbonyl O, the Nη1 binds the carbonyl O of His217, whereas the interaction distances with the Oδ atoms of Asp189 are relatively long. Similar to BPTI, the P2 Cys29-Cys48 disulfide bridge is located in the S2 pocket and rigidifies the reactive loop region together with the P3′ Cys33I-Cys50I disulfide, which adopts as the P2′ Arg a not-substrate-like position, in contrast to the P1′ Ile. Typical for canonical P3 and P4 residues, the side chain of His28I extends to the solvent and Val27I occupies the S4 subsite.