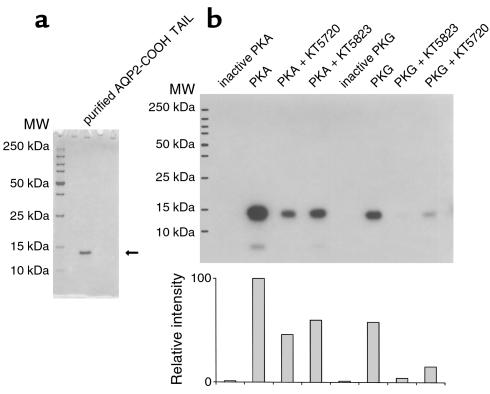
Figure 11.
In vitro phosphorylation of the AQP2 COOH tail by PKA and PKG. Purified AQP2 COOH tail was used as a substrate for the phosphorylation assay. The AQP2 COOH tail was purified and runs as a single band at 13 kDa on SDS-PAGE (a). Phosphorylation of this recombinant protein (b). It is phosphorylated by both PKA and PKG, and the phosphorylation is partially inhibited by 10 μM KT5720 and KT5823, respectively. However, these “specific” reagents also inhibit phosphorylation in crossover assays, although somewhat less effectively than when they are used to inhibit their “specific” target (PKA for KT5720 and PKG for KT5823). No AQP2 was phosphorylated when the PKA and PKG catalytic subunits were heat inactivated before inclusion in the assays (inactive PKA and inactive PKG). Neither PKA nor PKG phosphorylated the fusion protein in the absence of the AQP2 COOH tail (not shown). A quantification (by NIH Image software) of the degree of phosphorylation is shown in the lower panel of b.