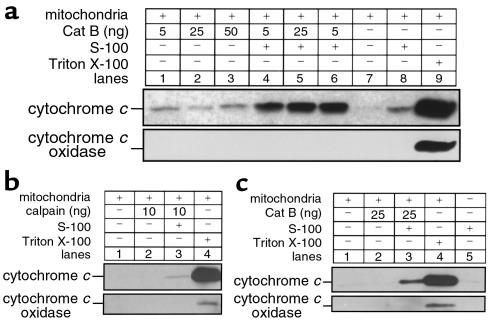
Figure 7.
Cat B–induced release of cytochrome c from mitochondria is enhanced by cytosol and is not due to a nonspecific proteolytic effect. Isolated mitochondria from catB+/+ mouse liver (25 μg protein) were incubated at 37°C with increasing concentrations of purified recombinant cat B (5–50 ng) (a) or m-calpain (10 ng) (b), in the presence or in the absence of S-100 cytosol fraction (50 μg) as described in Methods. Mitochondria were also treated with 0.1% Triton X-100 to induce maximum release of cytochrome c (positive control). After 1 hour, mitochondria were pelleted by centrifugation at 12,000 g for 5 min. Supernatants were subjected to SDS-PAGE on 15% acrylamide gels, transferred to nitrocellulose, and immunoblotted for cytochrome c. Blots were also probed for cytochrome c oxidase (subunit IV) to exclude mitochondria contamination in the supernatant. (c) Active cat B induces release of cytochrome c from isolated catB–/– mouse liver mitochondria in the presence of cytosol. Isolated mitochondria from catB–/– mouse liver (25 μg protein) were incubated at 37°C with purified recombinant cat B (25 ng), in the presence or in the absence of S-100 cytosol fraction (50 μg) obtained from the same animal. Mitochondria were also treated with 0.1% Triton X-100 to induce maximum release of cytochrome c (positive control). After 1 hour, mitochondria were pelleted by centrifugation at 12,000 g for 5 minutes, and the resulting supernatants were subjected to SDS-PAGE and subsequent immunoblot analysis for cytochrome c as already above. Immunoblot for cytochrome c oxidase (subunit IV) was also performed to exclude mitochondria contamination in the supernatant.