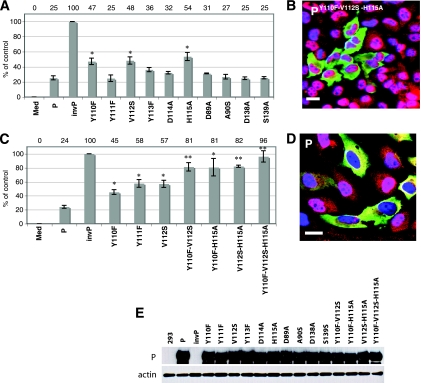
FIG. 2.
Three amino acids Y110, V112, and H115 contribute to the STAT1-inhibiting function of the P protein. (A and C) ISRE promoter activity after transfection with the plasmids indicated below each bar along with pISRE-luc and pCMV-RL. The firefly and Renilla luciferase activities, reflecting ISRE promoter activation and transfection efficiency, were measured. The y axis indicates the percentage of luciferase expression compared to the control plasmids (P and invP). The data represent the mean values ± the SD for triplicate samples. Med, medium; InvP, inverted P. One or two asterisks indicate statistical significance compared to the P control with a P < 0.05 and a P ≤ 0.001, respectively. (B and D) Nuclear translocation of STAT1. HeLa cells were transfected with an expression plasmid encoding the triple mutated P protein (B) or the standard P protein (D). After stimulation with IFN-α, the cells were fixed, permeabilized, and stained with antibody to P protein (green), STAT1 (red), and DAPI (blue). Merged sections of the three stains are shown. The overlap of red and green staining yields yellow, and the overlap of red and blue staining yields pink. Scale bar, 20 μm. (E) Immunoblot analysis of 293 cells transfected with the indicated plasmids. Membranes were blotted with antibodies against the phosphoprotein (P), as indicated on the left. Actin was used as a loading control.