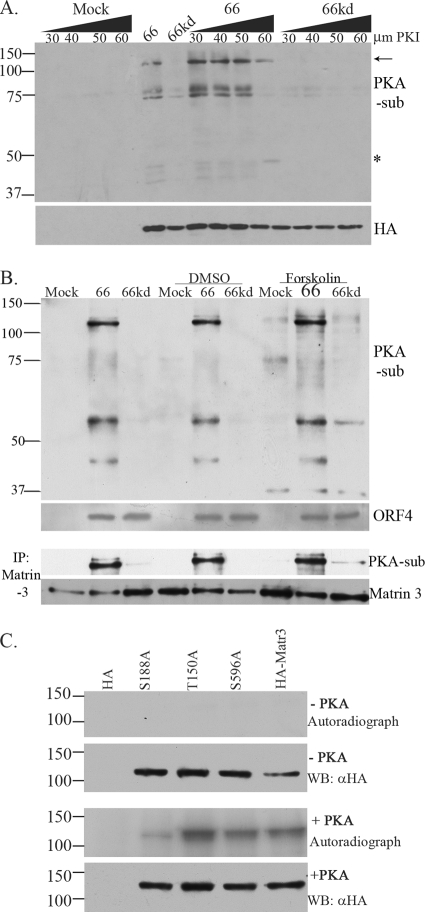
FIG. 6.
The 125-kDa matrin 3 is phosphorylated through a non-PKA pathway. (A) MRC-5 cells were pretreated with a PKA substrate inhibitor (PKI), 14-22 amide, 2 h prior to infection. Then cells either were infected with Ad.Toff at an MOI of 2.5 and with Ad.GFP-66 (66) or Ad.GFP-66kd (66kd) at an MOI of 5, with or without PKI, or were mock infected. Doses of PKI at increasing micromolar concentrations are given above the gel. Whole-cell extracts were immunoblotted. The membrane was first probed with a mouse α-HA antibody, stripped, and then reprobed with a rabbit α-PKA substrate antibody. The arrow indicates the 125-kDa PKA substrate (PKA-sub)/matrin 3 protein. The star indicates an undefined protein whose phosphorylation is PKI resistant. (B) MRC-5 cells either were infected with VZV.GFP-66 (66) or VZV.GFP-66kd (66kd) at an MOI of 0.1 or were mock infected. Where indicated, cells were treated with 10 μM forskolin or DMSO at the time of infection. Cells were harvested 1 day postinfection, analyzed by immunoblotting, and probed with a rabbit α-PKA substrate antibody. Viral protein expression was analyzed using a rabbit α-VZV ORF4 antibody. Cleared lysates were immunoprecipitated for matrin 3 and were subsequently analyzed by immunoblotting using rabbit α-PKA substrate; the blot was stripped and reprobed with a rabbit matrin 3 antibody. (C) In vitro kinase assay of immunoprecipitated HA alone, HA-tagged matrin 3 (HA-Matr3), or the HA-tagged S188A, T150A, or S596A matrin 3 mutant, incubated with PKA where specified (+ PKA). HA proteins were derived from transfected Hek 293T cells and were purified by immunoprecipitation using a mouse α-HA antibody. Following the kinase assay, proteins were separated on an SDS-PAGE gel, transferred to a PVDF membrane, and exposed to film. These membranes were then analyzed for HA fusion protein expression by probing with a mouse α-HA antibody. Protein size markers (in kilodaltons) are specified to the left.