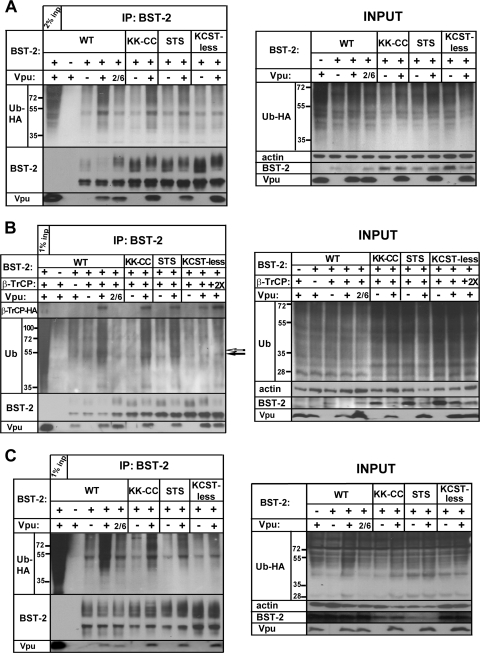
FIG. 8.
All potential acceptor sites in the CD of BST-2 are ubiquitinated by Vpu. (A) 293T cells were transfected, and BST-2 was immunoprecipitated as described in the legend of Fig. 1A (coexpression of ubiquitin-HA), except that the BST-2 mutants KK-CC, STS, and KCST-less were tested in addition to wild-type BST-2. The input samples shown in the right panel are the cell lysates before IP. The amount of actin in the input samples was assessed as a loading control. The immunoprecipitated proteins were probed for BST-2, ubiquitin-HA, and Vpu. The results shown are representative of two independent experiments. (B) 293T cells were transfected, and BST-2 was immunoprecipitated as described in the legend of Fig. 1B (coexpression of β-TrCP-HA) except that the BST-2 mutants KK-CC, STS, and KCST-less were tested in addition to wild-type BST-2. The immunoprecipitated proteins were probed for BST-2, endogenous ubiquitin, β-TrCP-HA, and Vpu. A band that is induced by Vpu but disappears in the case of KCST-less BST-2 is indicated by an open arrow; a band that is increased by Vpu but remains detectable in the case of KCST-less BST-2 is indicated by a filled arrow. The results shown are representative of two independent experiments. (C) 293T cells stably expressing BST-2 WT or the BST-2 mutants KK-CC, STS, and KCST-less were transfected with empty plasmid or Vpu- or Vpu2/6-expressing plasmid, along with a plasmid expressing ubiquitin-HA, and BST-2 was immunoprecipitated as described in Materials and Methods. The input samples shown in the right panel are the cell lysates before IP. The amount of actin in the input samples was assessed as a loading control. The results shown are representative of two independent experiments.