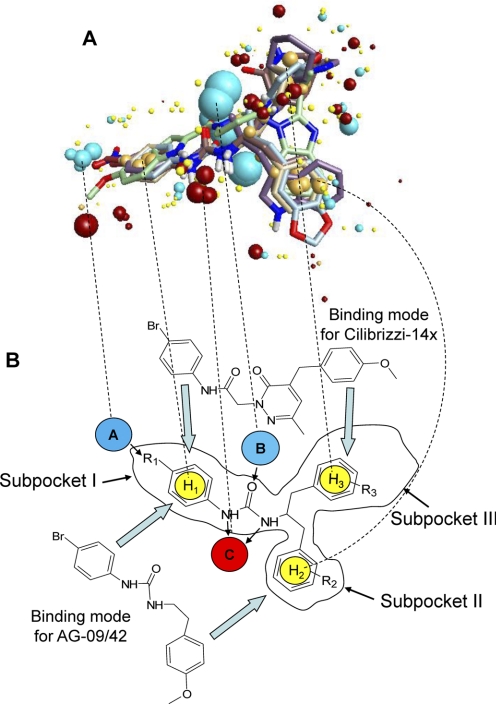
Fig. 5.
Multimolecule template for FPR2 and alignments of two molecules on the template. A, the multimolecule template was created using the best conformations of the following five molecules: AG-10/5, AG-10/8, AG-10/17, PD168368, and Frohn-11. Field points are colored as follows: blue, electron-rich (negative); red, electron-deficient (positive); yellow, van der Waals attractive (steric); and orange, hydrophobic. B, alignments for Cilibrizzi-14x and AG-09/42 in the template represent examples of two different modes of ligand-receptor interaction with the three hypothetical receptor subpockets I, II, and III. Arrows indicate directions of alignments for AG-09/42 in subpockets I/II and for Cilibrizzi-14x in subpockets I/III. Negative field points (blue spheres A and B) correspond to the receptor's positively charged regions (e.g., amino and hydroxyl groups in the active site that are capable of forming hydrogen bonds with electronegative atoms of the agonist). Positive field points (red sphere C) correspond to the receptor's negatively charged regions or to hydrogen bond acceptors in the FPR2 active site. Spheres H1, H2, and H3 correspond to hydrophobic centers. Substituents R1, R2, and R3 may influence lipophilicity, molar refraction, and atomic charges for respective groups of particular FPR2 agonists. Dashed lines show correspondences between centers of the main field points on the multimolecule template (A) and their schematic representations in B.