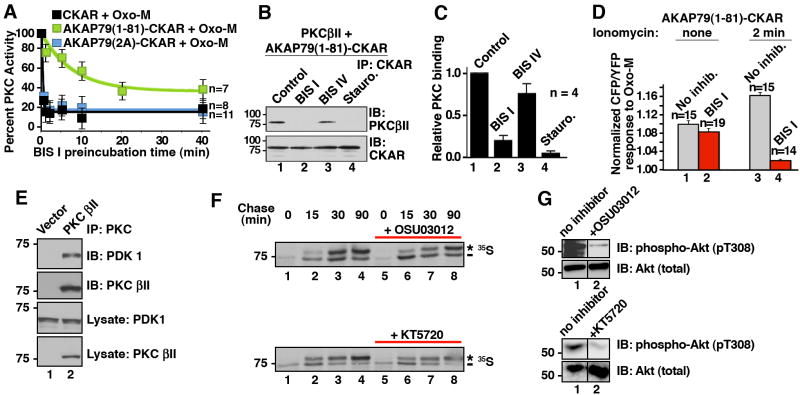
Figure 5. AKAP79 binding protects PKC from BIS I inhibition.
A) AKAP79 interaction alters PKC inhibition. Time course of FRET response to 3 μM Oxo-M: amalgamated data of cellular PKC activity measured from cells incubated with BIS I (500nM) for indicated times. Different reporters were used; CKAR (black, n=8), AKAP79(1-81)-CKAR (green, n=7) or AKAP79(2A)-CKAR (blue, n=11). Time points show peak PKC activity (CFP/YFP) 30 seconds after application of Oxo-M as percent of the response observed in the absence of BIS I treatment. B & C) Purified AKAP79(1-81)-CKAR was treated with PKC inhibitors (30 min) prior to addition of purified PKCβII. The binding is assessed by co-precipitating with AKAP79(1-81)-CKAR and PKCβII was identified by immunoblot. C) Amalgamated data from four co-IP experiments. PKC inhibitor competition with AKAP79 is shown as a decrease in relative PKC binding. D) In situ release of PKC from AKAP79(1-81)-CKAR was done by pretreatment of cells with ionomycin (2 min) prior to BIS I (500 nM, 5 min) and Oxo-M application. PKC activity was measured 30 sec after stimulation with Oxo-M. E) Co-immunoprecipitation of PDK1 with PKCβII. F) In pulse-chase experiments with 35S Met/Cys the ATP competitive PDK inhibitors, 10 μM OSU03012 or 1 μM KT5720, could not inhibit PDK-dependent phosphorylation of associated PKCβII. The asterisk indicates the mature, fully phosphorylated PKC and the dash indicates the newly synthesized, faster migrating PKC. G) The same concentration of OSU03012 and KT5720 inhibited PDK phosphorylation of a non-associated substrate, Akt1. Control immunoblots show cellular Akt protein levels are unchanged in the presence of inhibitors. N values and mean +/- SEM are indicated. (See also Figure S4.)