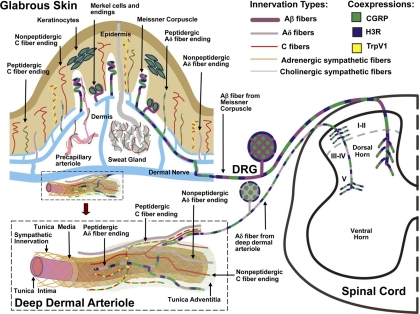
Fig. 1.
Schematic localization of H3Rs in the rat glabrous skin, DRG, and spinal cord. The innervation of glabrous skin is shown (top left), along with an enlargement of a deep dermal arteriole (bottom lower left). Terminations of H3R-expressing Aβ and Aδ innervation are shown in the dorsal horn of the spinal cord (right). Large-caliber (Aβ) and small-caliber (Aδ) myelinated fibers express 200-kDa neurofilament (purple). Smaller-caliber C fibers (red) lack neurofilament. CGRP (green) is expressed in certain types of C fibers, Aδ fibers, and keratinocytes. TrpV1 channels (yellow) are shown on certain CGRP-containing C fibers. Noradrenergic sympathetic fibers (orange) and cholinergic sympathetic fibers (gray) supply blood vessels and sweat glands, respectively. H3Rs (blue) are coexpressed with CGRP on Meissner Aβ fibers, Merkel cells, and keratinocytes (Cannon et al., 2007a). Although these components may be related to pain, the distribution of H3Rs on classically defined pain fibers is limited to the deep dermal perivascular Aδ fibers, which are closely associated with blood vessels. In the skin, activation of H3Rs on peptidergic Aδ fibers presumably suppresses neuropeptide release and attenuates inflammation-related edema (Ohkubo et al., 1995). In the spinal cord, activation of H3Rs reduces nociceptive responses elicited by low-intensity mechanical stimulation (Cannon et al., 2003) and proinflammatory agents such as formalin (Cannon et al., 2007b). Inhibition of transmitter release at the spinal terminations of these deep dermal fibers has been proposed to account for this antinociceptive activity. Although the figure is based on immunohistochemical studies of the skin, DRG, and spinal cord, H3R activation is known to reduce inflammatory peptide release the heart (Imamura et al., 1996), lung (Delaunois et al., 1995), and dura mater (Dimitriadou et al., 1997). Other spinal nerve terminals and spinal neurons may also have H3Rs.