Abstract
Arachidonic acid, a fatty acid component of neuronal cell membranes, forms the backbone of endogenous ligands of the endocannabinoid system. The lipid nature of this system may make it particularly susceptible to changes in fat content of the diet, which may, in turn, affect endocannabinoid tone and subsequent changes in receptor expression or activity. The latter would also be expected to affect responses to exogenous cannabinoids. The purpose of the present study was to determine the effects of a high-fat diet on sensitivity to the pharmacological effects of Δ9-tetrahydrocannabinol (Δ9-THC). Male and female Long-Evans rats were fed either a diet of standard rodent chow or chow enhanced with corn oil. Subsequently, they were repeatedly assessed for Δ9-THC-induced hypomobility, catalepsy and hypothermia. Female rats that received the high fat diet beginning in adolescence or in adulthood became significantly less sensitive to the effects of Δ9-THC on motor behavior, but not its hypothermic effects, with faster development of decreased sensitivity in female rats that began the high-fat diet as adults. In contrast, diet-induced differences either did not occur, or were less pronounced, in male rats of both ages. After acute injection, brain and blood levels of Δ9-THC and its two primary metabolites were similar regardless of diet. Combined with the fact that diet differentially affected only some of the measures, these results suggest that pharmacokinetic differences cannot fully account for the effects of the high-fat diet on response to Δ9-THC. Further, these results suggest that dietary fat content may represent an important consideration in predicting the effects of marijuana in females.
1. Introduction
Under normal conditions, the brain reward system functions to provide pleasurable affective responses to resources necessary for survival (e.g., food, sex). The initial stages of substance abuse may be conceived of as a hijacking of this system, partly through stimulation of the system to an intensity that often exceeds that of natural reinforcers (Volkow et al., 2009). Interestingly, other forms of addictive behavior (e.g., compulsive eating, unrestrained gambling) may share this propensity for enhanced stimulation; however, emerging evidence suggests that continued exposure to the target of the addiction (e.g., drug of abuse, highly palatable diet) may also elicit a downward spiral of addictive behavior that has been associated with the transition from drug use to “affective dependence” (Cottone et al., 2009, Koob, 1999). Indeed, the relationship between food and substance abuse is complex and multi-dimensional. While anecdotal and empirical findings over a number of years have shown that some classes of abused drugs affect eating behavior [e.g., marijuana-induced “munchies,” therapeutic use of dronabinol for AIDS wasting syndrome, anorectic effect of amphetamine and other psychostimulants] (Bray, 1993, Woolridge et al., 2005), recent studies have focused on the ways in which dietary composition may contribute to the potency and/or efficacy of drugs of abuse (Carta et al., 2006, Loebens and Barros, 2003, Sevak et al., 2008). For example, Wellman and colleagues (2007) found that administration of a diet high in fat content inhibited acquisition of cocaine self-administration in rats. These reports emphasize the importance of further delineation of the effects of diet composition on drug pharmacology, a research enterprise that takes on additional significance when co-morbidity among substance abuse, eating disorders, and psychiatric disorders is considered (Deas and Brown, 2006, Pisetsky et al., 2008).
The purpose of the present study was to determine the effects of a high-fat diet on sensitivity to the pharmacological effects of Δ9-tetrahydrocannabinol (Δ9-THC). In this study, the fat content of regular rodent chow was enhanced with corn oil such that it approximated the heightened levels of fat consumption that characterize the diet of Americans. Δ9-THC was chosen as a pharmacological probe for two reasons. First, it is the primary psychoactive substituent of marijuana, an illicit drug that has a high frequency of use, especially among adolescents who develop substance abuse problems. Second, the primary neural mechanism for Δ9-THC-induced behavioral alterations is agonist activity at brain cannabinoid (CB1) receptors, a component of the lipid-based endocannabinoid system. The lipid composition of endocannabinoids (e.g., anandamide and 2-arachidonoyl glycerol) may make this system particularly susceptible to changes in dietary fat content, which can directly and indirectly influence endocannabinoid levels (Artmann et al., 2008, Berger et al., 2001, Watanabe et al., 2003). In addition, previous studies have demonstrated intimate involvement of the endocannabinoid system in appetite regulation and eating behavior as well as in metabolic processes such as fat storage and distribution (for a review, de Kloet and Woods, 2009). Since adolescence is the developmental stage most often associated with initiation of substance abuse, the first two experiments described herein were initiated near the start of adolescence, which in rats typically ranges from postnatal day (PD) 28 to PD40 (Spear, 2000).
2.0 Methods
2.1 Subjects
Male and female Long-Evans rats were ordered from a commercial breeder (Harlan, Dublin, VA) as juveniles aged PD22 – PD25 [Experiments 1–4] or as adults (> PD65) [Experiment 5]. A total of 47 rats were used for the entire study: 17 male adolescent rats in Experiment 1, the same 18 female adolescent rats in Experiments 2–4, and 12 adult rats in Experiment 5. Upon arrival, rats were housed singly in clear plastic cages in a temperature-controlled (20°C–22°C) environment with 12-hour light and dark cycle (lights on at 6:00 am). They were allowed at least 3 days to habituate before testing. Throughout the experiment, all rats remained in their home cages when not being tested and had free access to water and the assigned food diet. The studies reported in this manuscript were carried out in accordance with guidelines published in the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996) and were approved by the Institutional Animal Care and Use Committee at Virginia Commonwealth University.
2.2 Diet
The high-fat diet used in these experiments was made by enriching standard rodent chow (Harlan Teklad Diet LM-485, Dublin, VA) with corn oil. According to manufacturer’s data (http://www.harlan.com/research_models_and_services/laboratory_animal_diets/teklad_traditional_diets/rodent_diets/teklad_lm485_mouse_rat_sterilizable_7012.hl), the standard diet contains 3.1 kcal/g metabolizable energy, with crude oil representing 5.7% of the content and 17% of the calories from fat. To obtain the high-fat diet, the standard rodent chow was soaked in room-temperature corn oil (20–22°C) for 24 h before being drained and dried on clean paper towels. Once prepared, the diet was sealed in plastic bags immediately and stored at 4°C for no more than 7 days. An initial experiment showed that 31 g of chow weighed 34.2 g after the enrichment process, resulting in enriched chow with 3.65 kcal/g and 36% fat calories. Since daily food intake was not measured, the amount of food consumed by rats in each group is uncertain. The food for the control animals was kept in a sealed container that was maintained at room temperature (20–22°C) until dispensed into the feeding hopper in the home cage. In order to control for inter-group differences in the freshness of the food, the standard chow was replenished at the same rate as the high-fat chow (i.e., once every 7 days).
2.3 Drugs
Δ9-THC was obtained from the Drug Supply Program of the National Institute on Drug Abuse (Rockville, MD) and was dissolved in a solution of Tween 80 (7.8%) and physiological saline (92.2%). Haloperidol (McNeil Pharmaceutical, Spring House, PA) was prepared by adding saline to a commercially available 5 mg/ml stock solution containing 1.8 mg methyl paraben, 0.2 mg propylparaben, and lactic acid. Morphine sulfate (NIDA) and d-amphetamine HCl (NIDA) were dissolved in physiological saline. O-1812 [(R)-(20-cyano-16,16-dimethyl docosa-cis-5,8,11,14-tetraenoyl)-1′-hydroxy-2′-propylamine] (Organix, Inc., Woburn, MA) and O-1839 [17-dimethyl-5,8,11,14-all-cis-docosatetraenoyl vanillylamide] (Organix, Inc.) were mixed in a vehicle of emulphor:ethanol:saline in a 1:1:18 ratio. All injections were administered intraperitoneally (i.p.) at a volume of 1 ml/kg, with the exception that the 70 mg/kg dose of Δ9-THC was adjusted from a 35 mg/ml solution and administered at a volume 2 ml/kg.
2.4 Apparatus
Clear plastic cages (44 cm in length X 22.5 cm in width X 20 cm in height) covered with ventilated lids and housed in sound-attenuating cabinets were used as locomotor chambers. The chambers did not contain bedding and were cleaned with an alcohol solution between sessions. The sessions were conducted in near dark conditions as the cabinet doors were closed. A cage rack system with photocell beams along the x-axis (8 beams) and y-axis (4 beams; Lafayette Instrument, Lafayette, IN) were placed around each chamber and positioned 4.5 cm above the chamber floor. Locomotor activity was measured as total number of beams broken during the 5-minute session.
A Traceable® digital thermometer (Control Company, Friendsville, TX) equipped with a flexible, plastic-coated probe was used to measure rectal temperature. Probes measuring 1.5 mm in diameter were used for determining temperatures in adolescent rats (e.g., PD30 and PD44) and probes 3.5 mm in diameter were used for adult rats (e.g., PD68 and PD114). A radiant-heat, tail-flick analgesia meter (Model #1430, Columbus Instruments, Columbus, OH) was used to assess antinociception.
The bar apparatus that was used to measure catalepsy-like behavior consisted of a 280-mm long bolt (12.5 mm in diameter) that was attached to a vertical frame by eyebolts. The height of the bar was adjusted based upon the age of the rat (80 mm high at PD30, 100 mm high at PD44, and 130 mm at >PD65). To reduce the influence of extraneous stimuli, each bar apparatus was enclosed on the back, top and sides. The cardboard enclosure was open in the front to permit access for the experimenter.
2.5 Procedure
2.5.1 In vivo testing procedure
Unless otherwise indicated for a particular experiment, procedural details for the assays included in each test session (i.e., locomotor activity, rectal temperature and catalepsy) are described in this section. In order to obtain a full dose-effect curve in each rat during every test session, Δ9-THC was administered in a cumulative dosing protocol with injections of 0, 3, 7, 20 and 70 mg/kg for total cumulative doses of 0, 3, 10, 30 and 100 mg/kg, respectively. The cumulative dosing sessions began with the determination of body weight and baseline body temperature followed by an i.p. injection of vehicle. Temperature was measured by inserting a rectal thermometer probe 40 mm (adolescents) or 45 mm (adults) into the rectum. The probe was dipped in mineral oil maintained at 37°C for lubrication before insertion into the rectum. The rats were then returned to their home cages for 20 min. Subsequently, they were placed in a locomotion chamber for 5 min. Locomotor activity was operationally defined as the total number of beam breaks during the entire 5-min session.
At the end of the locomotion session, body temperature was measured again followed immediately by placement of the rat on the bar apparatus for 5 min. During the 5-min session, the total amount of time (in s) that the front paws of a rat remained in contact with the bar was recorded. If both of the paws dropped from the bar, the animal was repositioned as before. The session timer was stopped briefly while the animal was repositioned. If the rat voluntarily removed both paws from the bar 10 times during the session, the session was terminated and the amount of time spent with both paws on the bar was recorded as “zero”. Each rat was tested in all three of these procedures. The total amount of time required for these measures was no more than 15 min (i.e., 5 min in the locomotion chamber plus 5 min on the bar apparatus plus the brief time required for measurement of body temperature).
Immediately after the end of the bar apparatus session, the rats were injected with a 3 mg/kg dose of Δ9-THC and returned to their home cage. When 20 min had elapsed, the entire triad of measures was repeated. These measures were taken 3 more times after the rats received successive injections of 7 mg/kg, 20 mg/kg and 70 mg/kg Δ9-THC. This cumulative dosing procedure allowed generation of a full dose-effect curve (vehicle control plus 3–100 mg/kg Δ9-THC) for each rat at each age point.
2.5.2 Experiment 1: Longitudinal effects of Δ9-THC in male adolescent rats fed a standard or high-fat diet
After habituation to the animal facility, male Long-Evans rats were randomly assigned to one of the two feeding conditions: standard rodent chow (n=8) and high-fat diet (n=9). On PD30 (pre-diet baseline), all rats in both groups were tested with cumulative doses of Δ9-THC in the in vivo testing procedure (see section 2.5.1). Subsequently, both groups received unlimited access to their assigned diet for the remainder of the study. Using the same procedure as for PD30, cumulative Δ9-THC dose-effect curves were re-determined on PD37, PD44, PD61, PD68, and PD114.
2.5.3 Experiment 2: Longitudinal effects of Δ9-THC in female adolescent rats fed a standard or high-fat diet
Group assignment (n=9 per feeding condition) and testing procedure for female rats were identical to that used for the male rats, with a couple of exceptions. The first exception was that female rats were tested with cumulative doses of Δ9-THC in the triad four times over the course of development (PD30, PD44, PD68, and PD114) whereas males were tested seven times. A second difference was that female rats were assessed in an antinociception assay on PD128. For this assay, the animals were injected with vehicle and returned to their home cage. After 30 min, latency to flick their tail away from the heat source was measured. Immediately after this baseline response was recorded, the animals were injected with 30 mg/kg Δ9-THC and again returned to their home cage. After another 30 min had elapsed, a second tail-flick measurement was taken. In order to prevent undue damage to the tail, the heat was turned off if the rat did not remove its tail before a 10-s maximum latency. In addition, rats received only two exposures to the tail-flick apparatus.
2.5.4 Experiment 3: Assessment of the effects of selected drugs on motor behavior in adult female rats fed a standard or high-fat diet since adolescence
The purpose of Experiment 3 was to determine whether different dietary fat content would produce differences in sensitivity to the motor effects of drugs other than Δ9-THC in female rats. Rats used in this experiment were the same rats as used in Experiment 2. These rats continued to be fed on the diet to which they had been assigned on PD30. Drugs selected for testing were amphetamine (a psychomotor stimulant), haloperidol (an antipsychotic and D2 receptor antagonist), morphine (an opioid), O-1812 (a metabolically stable analog of the endogenous cannabinoid anandamide) and O-1839 (a hybrid CB1/TRPV1 receptor ligand). Inter-injection intervals for each drug were the same as used for Δ9-THC. Although body temperature was measured at each dose, these data are not presented in the results section, as between-diet differences did not occur for these drugs or for Δ9-THC in the previous experiments.
2.5.5 Experiment 4: Measurement of Δ9-THC, 11-nor-Δ9-tetrahydrocannabinol-9-carboxylic acid (THC-COOH), and 11-hydroxy-Δ9-tetrahydrocannabinol (11-OH-THC) in blood and brain of adult female rats fed a standard or high-fat diet since adolescence
Measurement of Δ9-THC, THC-COOH, and 11-OH-THC occurred at two different time points. The first assessment was completed on or about PD205 and included evaluation of blood levels only. For this assessment, fourteenrandomly selected female rats from Experiment 2 (6 from the control diet group and 8 from the high-fat diet group) were treated with a 10 mg/kg dose of Δ9-THC (i.p.) and returned to their home cages. The remaining 4 rats were treated with vehicle and returned to their home cages. Thirty minutes after treatment with Δ9-THC, each rat was sedated with isoflurane and 0.2 ml of blood was withdrawn from the right saphenous vein with a heparinized syringe. Technical considerations unrelated to the experimental protocol prevented the withdrawal of blood from one of the rats that had been maintained on the control diet. Rats were returned to their home cages after they had recovered from sedation. After collection, blood was placed in 1.5-ml test tubes and stored on ice immediately. The samples remained on ice until blood was drawn from each rat. Subsequently, blood was processed as described below for the second measurement.
The second measurement occurred at the end of the study and included measurement of both blood and brain levels of Δ9-THC and its metabolites. For this experiment, rats were injected i.p. with 30 mg/kg Δ9-THC. Twenty minutes later 1 ml of blood was collected from the heart in a 1.5-ml heparinized test tube followed immediately by decapitation and placement of the head on ice until the brains could be removed. The brain tissues and blood samples were frozen at −80ºC after collection. Δ9-THC, 11-OH-THC and THC-COOH were extracted using a method, modified from Foltz et al. (1983). Deuterated internal standards (Cerilliant, Round Rock, Texas) were added to each homogenized brain tissue or blood sample. Following overnight equilibration, 2 mL of ice cold acetonitrile was added drop by drop to each samples while vortexing. The samples were then centrifuged at 3500 rpm for 10 min. After centrifuging the samples were placed in −40°C freezer for at least two hours. The top layer containing the acetonitrile was removed via a disposable glass pipette and placed in clean test tube. The acetonitrile was dried under nitrogen. Samples were reconstituted with 100 μL of acetonitrile and placed in autosampler vial for LC-ESI-MS-MS analysis.
Resolubilization of Δ9-THC/deuterated Δ9-THC was achieved in 0.1 ml methanol (HPLC grade, Fisher Scientific) followed by LC-MS quantification (3200 QTRAP, Applied Biosystems, Foster City, CA). For LC-MS, the mobile phase consisted of water/methanol (10:90 v:v) with 0.1mM ammonium formate and was delivered at a flow rates of 0.5 mL/min. A guard column was used inline with the standard reverse phase C18 column (Zorbaz eclipse XDB-C18 column, Agilent Technologies, USA). The mass spectrometer was run in APCI+ mode. A calibration curve was constructed for each assay based on linear regression using the separate peak-area ratios of Δ9-THC, 11-OH-THC and THC-COOH to respective deuterated compounds of the extracted calibration samples. No peaks were observed for any of the blank samples.
2.5.6 Experiment 5: Effects of Δ9-THC in adult male and female rats fed a standard or high-fat diet
Although rats in previous experiments were assessed repeatedly during adolescence and adulthood, they were all fed their assigned diet beginning in early adolescence (PD30) and continued on this diet for the rest of their lives. Hence, observed effects could have been a function of the high-fat diet alone or initiation of this diet during adolescence. The purpose of this experiment was to determine the importance of initiation of a high-fat diet specifically during adolescence. Male and female rats received a diet of standard rodent chow until they were adults (> PD65). After habituation to the animal facility, each adult rat was assessed in the same triad of pharmacological tests used in previous experiments with adolescent rats. Subsequently, they were randomly assigned to continue a diet of standard rodent chow or to receive a high-fat diet (n=3/group for each sex). All rats were tested in the triad battery two weeks later.
2.6 Data analysis
Spontaneous activity was measured as total number of photocell beam interruptions during each 5-min test. Rectal temperature values were expressed as the difference between control temperature (before injection) and temperatures following each cumulative drug administration (Δ°C). During placement on the bar apparatus, the total amount of time (in s) that the rat had both front feet in contact with the bar was measured and was used as an indication of catalepsy-like behavior. This value was divided by 300 s and multiplied by 100 to obtain a percent time on bar rating. For each of these three dependent measures, means (± SEM) were calculated at each dose and time test and for each sex and age separately. For Experiments 1–3, separate mixed factorial ANOVAs (diet X cumulative dose) were performed on each of the dependent measures at each PD. Body weights for adolescents of each sex were also analyzed with separate mixed factorial ANOVAs (diet X day). For the antinociception measure included in Experiment 2, latency to remove the tail from the heat source was recorded. After vehicle injection, latencies were divided by the maximal possible latency (10) and converted to a percentage. After injection of 30 mg/kg Δ9-THC, latencies were expressed as percent maximal possible effect by using the formula {(latency after Δ9-THC - latency after vehicle)/(10 – latency after vehicle)} and conversion of the result to a percentage. Data from Experiment 4 were analyzed with t-tests comparing levels of Δ9-THC and each of its metabolites in rats fed standard versus high-fat diets. For Experiments 5 and 6, separate mixed factorial ANOVAs (diet X cumulative dose X time) were performed on each of the dependent measures. Significant 3-way interactions were further analyzed through the use of separate mixed factorial ANOVAs (diet X cumulative dose) at each time point. When any ANOVA was significant, Tukey-Kramer post-hoc tests (α=0.05) were used to compare individual means.
3.0 Results
3.1 Experiment 1
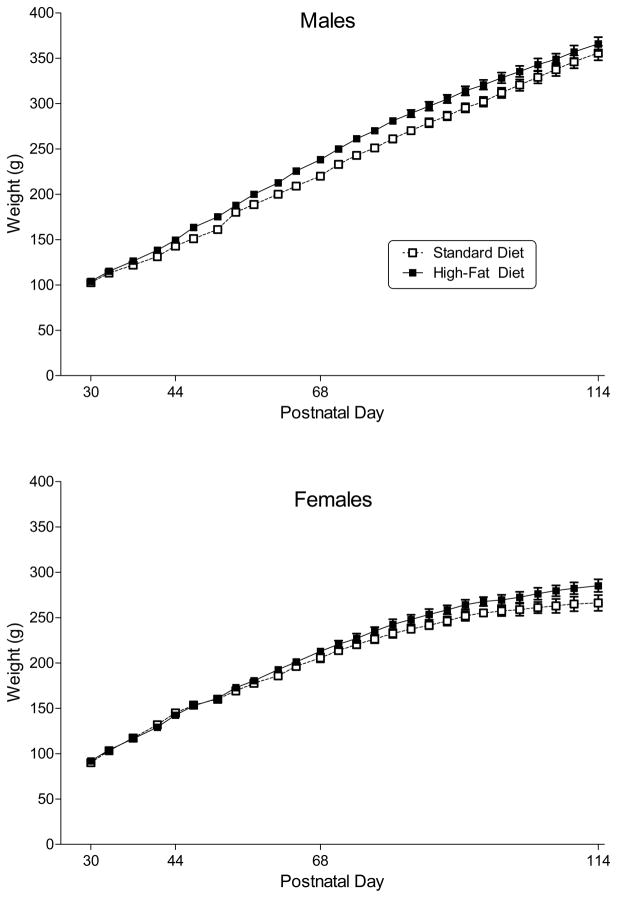
All male rats gained a substantial amount of weight over the course of adolescence (Fig. 1, top panel) [main effect of PN day: F(26, 390) = 671.4, p < 0.001]. In addition, male rats that were fed a high-fat diet weighed significantly more than those fed a standard diet [main effect of diet: F(1, 15) = 126.8, p < 0.001].
Figure 1.
Body weight (in g) across development in male and female rats (top and bottom panel, respectively) fed a standard or high-fat diet during adolescence and early adulthood. Open squares represent mean (± SEM) for rats fed a standard diet (n=8–9). Filled symbols represent mean (± SEM) for rats fed a high-fat diet (n=9). Main effects for postnatal day and for diet were significant for rats of both sexes; however, day X diet interaction was not significant for either sex.
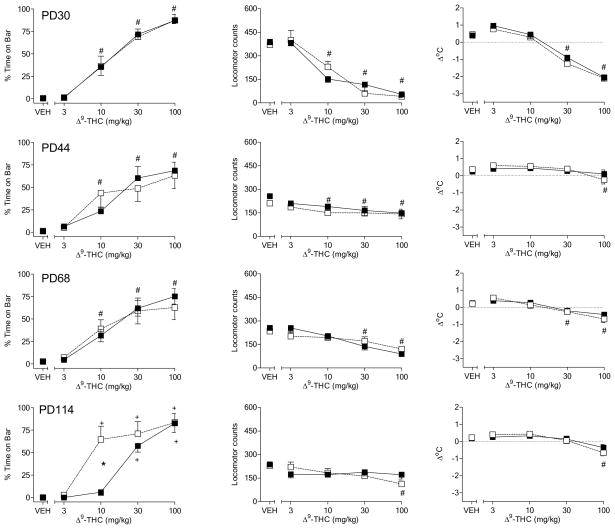
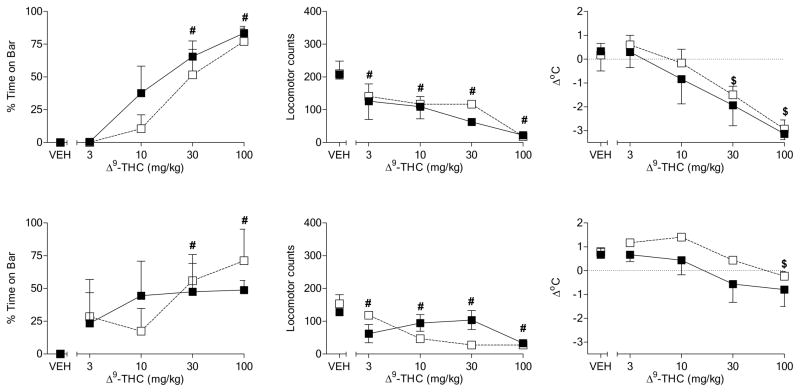
Figure 2 shows the pharmacological effects of Δ9-THC in male rats fed a standard or high-fat diet throughout adolescence and early adulthood. Before initiation of the different diets on PD30, Δ9-THC dose-dependently affected both measures of motor function by increasing the amount of time with forepaws on the bar in the bar test and decreasing spontaneous activity (Fig. 2, top left and middle panels, respectively) [dose main effects: F(4,60)=104.9, p< 0.05 and F(4,60) = 66.7, p<0.05, respectively]. In addition, Δ9-THC dose-dependently decreased body temperature (Fig. 2, top right panel) [dose main effect: F(4,60) = 96.0, p<0.05]. Pre-diet between-group differences in the effects of Δ9-THC were not observed for any measure. During subsequent assessments on PD44 and PD68, Δ9-THC continued to produce dose-dependent effects in all three pharmacological assays, without significant differences between the diet groups (Fig. 2, 2nd and 3rd row panels). Visual inspection revealed that Δ9-THC dose-effect curves for spontaneous activity and body temperature were flattened in test sessions subsequent to the pre-diet assessment, suggesting the development of tolerance for these two measures. Although male rats were also tested on PD37 and PD61, data for these tests did not differ substantially from data obtained on PD30, PD44 and PD68. Hence, for consistency of presentation, only data from days on which female adolescent rats were also tested (see Experiment 2) are presented here.
Figure 2.
Effects of cumulative doses of Δ9-THC on catalepsy-like behavior (% time with forepaws on bar) [left panels], locomotor counts (middle panels) and change in rectal temperature (right panels) in male rats on PD30 (top row of panels), PD44 (2nd row of panels), PD68 (3rd row of panels), and PD114 (bottom row of panels). Open squares represent mean (± SEM) for rats fed a standard diet (n=8). Filled symbols represent mean (± SEM) for rats fed a high-fat diet (n=9). # indicates significant main effect of dose. * indicates significant interaction and post-hoc between-diet difference (p<0.05). + indicates significant interaction and post-hoc difference (p<0.05) of dose compared to vehicle for the indicated diet group.
Similar to its effect in previous assessments, on PD114 Δ9-THC decreased spontaneous activity and body temperature [dose main effects: F(4,60) = 4.5, p<0.05 and F(4,60)=26.5, p< 0.05, respectively], albeit the maximum magnitude of these effects was smaller than pre-diet baseline (Fig. 2, bottom middle and right panels). Differences between diet groups for these measures were not observed. In contrast, males rats fed a high-fat diet spent significantly less time with forepaws on the bar following injection of 10 mg/kg Δ9-THC as compared to rats that received the standard diet (Fig. 2, bottom left panel) [diet X dose interaction: F(4,60) = 8.2, p<0.05]. Further, rats in the standard diet group exhibited an increase in bar time (compared to vehicle) at this dose whereas rats in the high-fat diet group did not. However, maximum magnitude of this measure for the two diet groups at higher doses did not differ.
3.2 Experiment 2
All female rats gained a substantial amount of weight over the course of adolescence (Fig. 1, bottom panel) [main effect of PN day: F(26, 416) = 301.2, p < 0.001]. In addition, female rats that were fed a high-fat diet weighed significantly more than those fed a standard diet [main effect of diet: F(1, 16) = 37.5, p < 0.001].
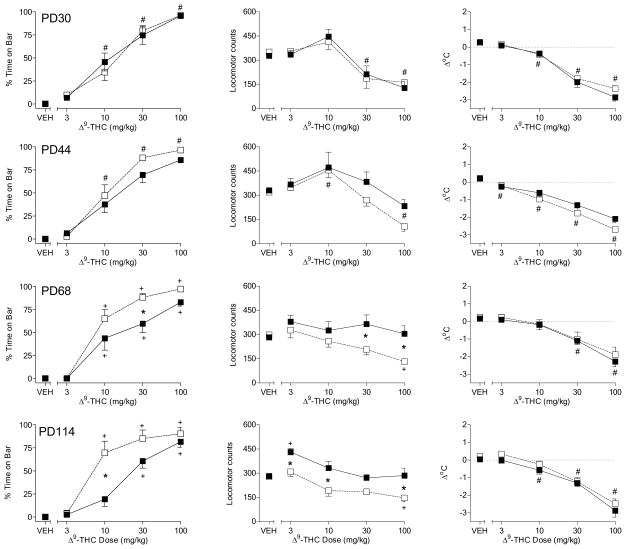
In adolescent female rats (PD30), Δ9-THC produced dose-dependently increased the amount of time with forepaws on the bar apparatus, decreased spontaneous activity, and decreased body temperature (Fig. 3, top left, middle and right panels, respectively) [dose main effects: F(4,64)=150.1, p< 0.05, F(4,64) = 23.8, p<0.05, and F(4,64)=144.0, p< 0.05, respectively]. Pre-diet differences between groups were not observed for any of the measures. On PD44, Δ9-THC continued to produce all three effects in a dose-dependent manner [dose main effects: F(4,64)=123.2, p< 0.05, F(4,64) = 14.1, p<0.05, and F(4,64)=99.6, p< 0.05, respectively], with little change in the maximal magnitude of any measure as compared to PD30 (Fig. 3, 2nd row panels). In addition, between-diet differences were not evident. On PD68, however, sensitivity to Δ9-THC-induced motor effects (i.e., time on bar and spontaneous activity) was decreased in female rats fed a high-fat diet throughout adolescence as compared to those fed a standard diet (Fig. 3, 3rd row, left and middle panels, respectively) [diet X dose interactions: F(4,64)=2.7, p< 0.05 and F(4,64) = 4.2, p<0.05, respectively]. Although the effects of Δ9-THC on spontaneous activity and bar time were significantly less pronounced at one or more doses in rats fed a high-fat diet, the maximal magnitude of Δ9-THC-induced increase in bar time was similar in both groups. In contrast, Δ9-THC produced a significantly larger decrease in spontaneous activity in rats fed a standard diet than in those fed a high-fat diet. Further, rats fed a high-fat diet were visibly more alert and active after administration of 10–100 mg/kg Δ9-THC than were rats fed a standard diet. While both groups of rats kept their forepaws on the bar during a significant portion of the session, rats in the high-fat diet moved horizontally on the bar whereas rats in the standard diet group remained immobile. Hence, larger between-group differences would have occurred had a different operational definition of “catalepsy” been adopted. Notably, however, the effects of the same doses of Δ9-THC on body temperature were similar between diet groups (Fig. 3, 3rd row, right panel). The decreased sensitivity of the high-fat group to the motor effects of Δ9-THC (Fig. 3, bottom left and middle panels) [diet X dose interactions: F(4,64) = 3.3, p<0.05 and F(4,64)=6.9, p< 0.05 for locomotor activity and the bar test, respectively], but not its effects on temperature (Fig. 3, bottom right panel), was also evident on PD114 and occurred at lower doses.
Figure 3.
Effects of cumulative doses of Δ9-THC on catalepsy-like behavior (% time with forepaws on bar) [left panels], locomotor counts (middle panels) and change in rectal temperature (right panels) in female rats on PD30 (top row of panels), PD44 (2nd row of panels), PD68 (3rd row of panels), and PD114 (bottom row of panels). Open squares represent mean (± SEM) for rats fed a standard diet (n=9). Filled symbols represent mean (± SEM) for rats fed a high-fat diet (n=9). # indicates significant main effect of dose. * indicates significant interaction and post-hoc between-diet difference (p<0.05). + indicates significant interaction and post-hoc difference (p<0.05) of dose compared to vehicle for the indicated diet group.
On PD128, female rats were assessed for antinociception in the tail flick apparatus (Table 1). Diet-induced differences in percent maximal possible latency were not observed following vehicle injection; however, after injection with 30 mg/kg Δ9-THC, female rats that had been fed a high-fat diet showed less antinociception than did those fed a regular diet [diet X treatment interaction: F(1,16) = 9.5, p<0.05].
Table 1.
Antinociceptive effects of Δ9-THC in female rats fed a standard or high-fat diet.
| Treatment | Standard Diet | High-Fat Diet |
|---|---|---|
| Vehicle | 28 ± 2.9 | 36 ± 5.1 |
| Δ9-THC (30 mg/kg) | 70 ± 5.3 + | 57 ± 2.2 +* |
Antinociception is expressed as percentage of maximum possible effect in a tail flick assay. Each value represents the mean (± SEM) of data from 9 rats.
indicates significant difference from corresponding vehicle condition.
indicates significant difference compared to standard diet.
3.3 Experiment 3
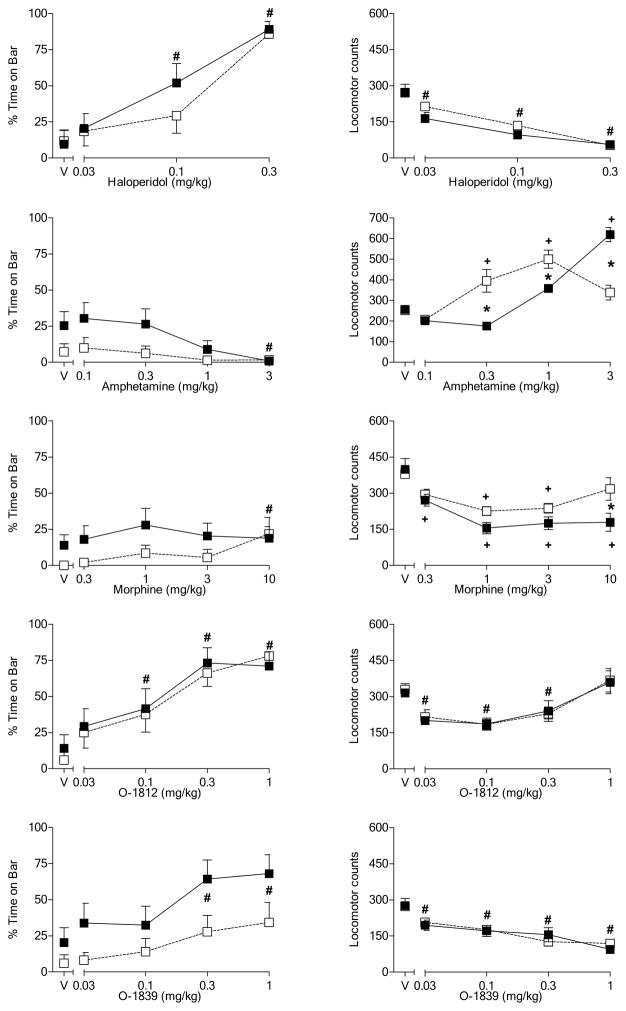
Figure 4 shows the effects of selected drugs on the motor behavior: spontaneous activity (left panels) and time with forepaws on the bar apparatus (right panels). Drug-induced body temperature changes, if present, tended to be of small magnitude and did not differ between diet groups (data not shown). With the exception of amphetamine (Fig. 4, 2nd row, left panel), all of the drugs decreased activity at one or more doses [dose main effects: haloperidol, F(3,48) = 55.2, p<0.05; amphetamine, F(4,64) = 32.6, p<0.05; morphine, F(4,64) = 21.1, p<0.05; O-1812, F(4,64) = 21.1, p<0.05; O-1839, F(4,64) = 33.6, p<0.05]. Haloperidol (Fig. 4, top right panel), O-1812 (Fig. 4, 4th row, right panel), and O-1839 (Fig. 4, bottom right panel) also significantly increased bar time [dose main effects: haloperidol, F(3,48) = 52.7, p<0.05; O-1812, F(4,64) = 28.7, p<0.05; O-1839, F(4,64) = 12.3, p<0.05]. Interestingly, amphetamine also produced the most pronounced diet-induced differences in spontaneous activity [diet X dose interaction: F(4,64) = 21.4, p<0.05]. Indeed, with the exception of a small (but significant) enhancement of the activity decreasing effects of 10 mg/kg morphine in rats fed a high-fat diet (Fig. 4, 3rd row, left panel) [diet X dose interaction: F(4,64) = 2.9, p<0.05], none of the other drugs differentially affected either measure of motor functioning. Unlike the other drugs, amphetamine significantly increased spontaneous activity in both diet groups; however, it did so at different doses. In rats fed a standard diet, these increases were observed at lower doses of 0.3 and 1 mg/kg with a decrease to vehicle levels at the 3 mg/kg dose. In contrast, increases in spontaneous activity were observed in rats fed a high in fat diet only at the highest (3 mg/kg) dose, suggesting that this group was not as sensitive to the stimulant effects of amphetamine.
Figure 4.
Effects of cumulative doses of haloperidol (top panels), amphetamine (2nd row of panels), morphine (3rd row of panels), O-1812 (4th row of panels) and O-1839 (bottom row of panels) on catalepsy-like behavior (% time with forepaws on bar) [left panels] and locomotor counts (right panels) in female rats previously tested with Δ9-THC (see Figure 2). Open squares represent mean (± SEM) for rats fed a standard diet (n=9). Filled symbols represent mean (± SEM) for rats fed a high-fat diet (n=9). # indicates significant main effect of dose. * indicates significant interaction and post-hoc between-diet difference (p<0.05). + indicates significant interaction and post-hoc difference (p<0.05) of dose compared to vehicle for the indicated diet group.
3.4 Experiment 4
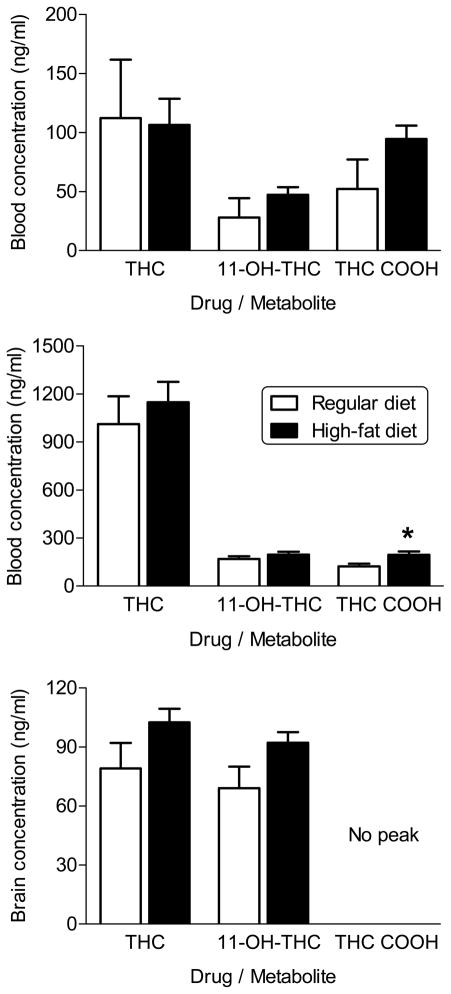
Figure 5 shows that the blood concentrations of Δ9-THC and its two major metabolites, 11-OH-THC and THC-COOH, following an injection of 10 mg/kg Δ9-THC on PD205 in female rats that received a high-fat diet throughout adolescence did not differ from those that were fed a regular diet during the same time period (Fig. 5, top panel). Non-systematic observations made during the recovery period suggest that female rats maintained on a high-fat diet took longer to recover from isoflurane sedation than did female rats maintained on the control diet. Subsequent to completion of the pharmacological testing in Experiments 2 and 3, diet-exposed female rats were injected with 30 mg/kg Δ9-THC prior to sacrifice. Terminal blood and brain concentrations of Δ9-THC and 11-OH-THC did not differ between diet groups (Fig. 5, middle and bottom panels, respectively). Concentrations of THC-COOH were not measurable in brain for either diet group (Fig. 5, bottom panel), but blood concentrations were significantly higher in the female rats fed a high-fat diet (Fig. 5, middle panel) [t(16) = −2.7, p<0.05]. However, the overall concentration of THC-COOH was low in both groups and the magnitude of difference was small.
Figure 5.
Effects of diet on blood and brain levels of Δ9-THC and its major metabolites, 11-OH-THC and THC-COOH, in female rats. Blood levels were assessed on PD205 following injection with 10 mg/kg Δ9-THC (top panel) and at completion of the study after injection with 30 mg/kg Δ9-THC (middle panel). Brain levels were assessed at the end of the study after injection with 30 mg/kg Δ9-THC (bottom panel). Open bars represent mean (± SEM) for rats fed a standard diet (n=9). Filled bars represent mean (± SEM) for rats fed a high-fat diet (n=9). * indicates significant difference of value for high-fat diet versus corresponding value for standard diet.
3.5 Experiment 5
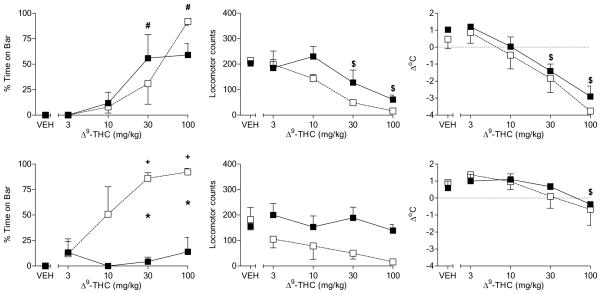
In order to assess age dependence of the dietary attenuation of Δ9-THC-induced motor effects in rats, adult rats of both sexes were evaluated in the pharmacological battery before and after initiation of a standard or high-fat diet. Before initiation of the diet, Δ9-THC decreased spontaneous activity and rectal temperature and increased time spent with forepaws on the bar in adult male rats (top panels, Fig. 6) [dose main effects: F(4,16) = 14.3, p<0.05, F(4,16) = 50.1, p<0.05, and F(4,16) = 18.4, p<0.05]. Further, neither sensitivity nor maximum magnitude of the effects differed across diet group assignment. After exposure to a high-fat diet for two weeks, adult male rats exposed to either diet showed approximately equal sensitivity to Δ9-THC-induced effects in each pharmacological assay (Fig. 6, bottom panels). Although the magnitude of Δ9-THC’s reduction of spontaneous activity and temperature in these rats was less than that seen before treatment, it did not differ between diet groups. Hence, the high-fat diet used here did not alter sensitivity to the effects of Δ9-THC, regardless of when it was initiated (i.e., during adolescence or adulthood).
Figure 6.
Effects of cumulative doses of Δ9-THC on catalepsy-like behavior (% time with forepaws on bar) [left panels], locomotor counts (middle panels) and change in rectal temperature (right panels) in male rats before initiation of assigned diet (top panels) and after two weeks on the diet (bottom panels). Open squares represent mean (± SEM) for rats fed a standard diet (n=3). Filled symbols represent mean (± SEM) for rats fed a high-fat diet (n=3). # indicates significant main effect of dose. $ indicates significant dose X time interaction and post-hoc difference between Δ9-THC dose and vehicle across diets at indicated time (p<0.05).
Figure 7 shows that the pattern of results produced by Δ9-THC in female adult rats before initiation of the high-fat diet resembled the pattern that occurred in naïve female adolescent rats and in male rats of both ages (i.e., dose-dependent decrease in spontaneous activity and rectal temperature and increase in time with forepaws on the bar) [Fig. 7, top panels]. Following a two-week exposure to a high-fat diet, however, the effects of Δ9-THC on motor behavior were attenuated as compared with effects in female rats fed a standard diet (Fig. 7, bottom panels) [main effect of diet for locomotor activity: F(1,16) = 9.0, p<0.05; diet X dose interaction at 2-week time point: F(4,16) = 7.6, p<0.05]. The effects of diet on Δ9-THC-induced hypothermia were difficult to determine, as significant tolerance to the initial effects of Δ9-THC on rectal temperature was observed with a resulting floor effect.
Figure 7.
Effects of cumulative doses of Δ9-THC on catalepsy-like behavior (% time with forepaws on bar) [left panels], locomotor counts (middle panels) and change in rectal temperature (right panels) in female rats before initiation of assigned diet (top panels) and after two weeks on the diet (bottom panels). Open squares represent mean (± SEM) for rats fed a standard diet (n=3). Filled symbols represent mean (± SEM) for rats fed a high-fat diet (n=3). # indicates significant main effect of dose. $ indicates significant dose X time interaction and post-hoc difference between Δ9-THC dose and vehicle across diets at indicated time (p<0.05). For catalepsy, significance symbols are used to specify differences revealed by follow-up 2-way ANOVAs. For this measure only, * indicates significant diet X dose interaction and post-hoc between-diet difference (p<0.05). + indicates significant diet X dose interaction and post-hoc difference (p<0.05) of dose compared to vehicle for the indicated diet group.
4.0 Discussion
The results of this study demonstrate that exposure to a diet high in fat decreases sensitivity to the motor, but not the hypothermic, effects of Δ9-THC. While this effect was seen in male rats exposed to the high-fat diet beginning in adolescence and continuing into adulthood, it was substantially more pronounced and occurred after fewer days of exposure in female rats. Several findings suggest that these effects are not likely to be entirely the consequence of between-diet differences in pharmacokinetics. First, blood levels of Δ9-THC and its major metabolites, 11-OH-THC and THC-COOH, were similar following an injection of 10 mg/kg Δ9-THC to female rats on or about PD205 and after an injection of 30mg/kg Δ 9-THC at the termination of the study. At this second time point, brain levels of Δ9-THC and 11-OH-THC were also similar across diet. Second, sensitivity to amphetamine-induced stimulation of locomotor activity was also altered in female rats fed a high-fat diet whereas the effects of haloperidol and morphine on motor activity were not. Because the cytochrome p450 system is involved in the metabolism of all of these drugs, but differences are present only for selected effects of Δ9-THC and amphetamine, overall alteration of the functioning of cytochrome p450 enzymes is not a likely explanation of between-diet differences in drug sensitivity. In addition, the primary families of p450 enzymes responsible for the metabolism of amphetamine and Δ9-THC, the two drugs that showed effects of the high-fat diet, differ [CYP2D versus CYP2C9 and CYP3A4, respectively; (Law et al., 2000, Yamamoto et al., 1995)]. Third, the decreased sensitivity to Δ9-THC-induced motor effects in rats fed the high-fat diet was in contrast with the lack of diet effects on Δ9-THC-induced hypothermia, a difference that would not be expected if changes in pharmacokinetics were entirely responsible for the observed effects of the diet on Δ9-THC pharmacology. Finally, while body weights of rats differed significantly across diet, these differences averaged less than 10% and occurred in both sexes, whereas differences in sensitivity were seen primarily in females.
The reductions in sensitivity to the motor, but not the hypothermic, effects of Δ9-THC in female rats fed a high-fat diet during adolescence observed here are reminiscent of the task specificity of cannabinoid cross-tolerance, with development of substantially less cross-tolerance to the hypothermic effects of anandamide and its analogs in Δ9-THC-tolerant mice as compared to their effects on locomotion, antinociception and catalepsy (Wiley et al., 2005). This pattern of selective differences is also consistent with reports of regional selectivity in changes in CB1 receptor binding resulting from maintenance on a diet high in fat: i.e., decreases in the striatum and other brain areas associated with motor behavior and reward and an absence of differences in the hypothalamus (Harrold et al., 2002), the most likely locus for mediation of Δ9-THC’s hypothermic effects. Similarly, chronic exposure to cannabinoids produces CB1 receptor downregulation and desensitization that is regionally selective and dependent upon ligands used for binding and chronic dosing (Sim-Selley, 2003). The ligand-dependent nature of downregulation and desensitization is a particularly important consideration, as female rats on the high-fat diet that did not exhibit decreased sensitivity to the anandamide analog O-1812. Additionally, this latter observation is consistent with prior findings of distinct pharmacological differences among the various classes of cannabinoids (for reviews, Thakur et al., 2005, Wiley and Martin, 2002); however, the possible contribution of differences in duration of exposure to the high-fat diet at the time of cannabinoid testing (O-1812 > Δ9-THC) also cannot be eliminated. Hence, the behavior of female rats fed a high-fat diet during adolescence resembles that of cannabinoid-tolerant rodents, including few overt differences from controls until presentation of an external challenge affecting CB1 receptors (e.g., Δ9-THC injection), and may have similar underlying neural mechanisms, albeit confirmatory evidence is still needed.
Perhaps the most prominent observation reported here, however, is sexual dimorphism of the effects of the high-fat diet. Female rats of both ages exhibited alterations in sensitivity to Δ9-THC-induced motor effects that occurred sooner and were greater in magnitude or scope than those seen in their male counterparts. Although an in-depth examination of the basis for these sex differences was beyond the scope of the present study, several points are worth noting. First, sex differences in the pharmacokinetics of Δ9-THC have been reported. Whereas 11-OH-THC is the principal metabolite of Δ9-THC in female rats, male rats show a higher proportion of other cannabinoids, as well as 11-OH-THC (Tseng et al., 2004). It is possible that these other cannabinoids may have modulated the effects of exposure to a high-fat diet in males. Second, the duration of exposure to the high-fat diet that was necessary for the appearance of decreased sensitivity to Δ9-THC’s motor effects was substantially shorter in adult females than in adolescent females, suggesting that developmental and/or hormonal factors may play modulatory roles. Others have reported sex- and age-dependent differences in the binding and/or functional status of CB1 receptors under various hormonal states or following administration of Δ9-THC (Burston et al., 2010, Rodriguez de Fonseca et al., 1994). The fact that similar CB1 receptor changes are produced by exposure to a high-fat diet raises the possibility that this type of diet may also differentially affect CB1 receptors dependent upon sex and age. Alternatively, a high-fat diet may differentially affect levels of endocannabinoids in males and females, with a possible indirect effect on CB1 receptor density or function. Previous work has demonstrated that certain high-fat diets have divergent effects on resulting fatty acid composition of lipids in plasma and liver in male and female rats (Childs et al., 2010), suggesting that brain lipids may also differ between males and females as a consequence of a high-fat diet. In addition, high-fat diets have been reported to affect other appetite regulatory systems (e.g., leptin and ghrelin) in a sexually dimorphic manner (Priego et al., 2009) and may affect levels of sex hormones (especially estrogen), which may, in turn, affect dietary intake (Butera, 2010). Indeed, the endocannabinoid system is only one among myriad systems that are affected directly or indirectly by a high-fat diet.
In the present study, sex differences in sensitivity to Δ9-THC were observed in a battery of relatively simple tests that did not require learning. Sex differences in cannabinoid pharmacology have also been reported in procedures more relevant to their abuse potential. For example, self-administration of the aminoalkylindole cannabinoid WIN 55,212-2 occurred more rapidly in female rats than in male rats (Fattore et al., 2007). Further, this difference was obviated by bilateral ovariectomy, suggesting hormonal mediation. Previous studies have demonstrated that female rats also exhibit greater sensitivity to the abuse-relevant effects of substances of abuse other than cannabinoids and to the process of transitioning from use to dependence (Carroll et al., 2004, Lynch et al., 2002, Roth et al., 2004). Again, these differences were sensitive to the hormonal status of the females.
While the mechanistic basis for the sex-dependent decreases in sensitivity to the motor effects of Δ9-THC following exposure to a high-fat diet (as reported in this study) remains uncertain, preliminary pharmacokinetic data do not support differences in drug metabolism as a causative factor. Previous findings that exposure to a high-fat diet alters CB1 receptor functioning in manner reminiscent of tolerance development following repeated cannabinoid administration hint that changes in functioning of the endocannabinoid system may play a role in the decreased sensitivity. Although sparse, studies that have examined CB1 receptor adaptation following repeated Δ9-THC administration in rats of both sexes have reported that females tend to show more pronounced changes. Further, these changes are affected by hormonal status. Together with these previous findings, the results reported here suggest that females who consume a diet high in fat may be less sensitive to the acute effects of marijuana and perhaps more likely to transition to chronic use, given that initial sensitivity has been shown to predict subsequent dependence on alcohol (Kramer et al., 2008), marijuana (Fergusson et al., 2003), and nicotine (Perkins et al., 2009).
Acknowledgments
The authors thank Katherine Nicholson, Qing Tao and Justin Poklis for technical assistance in conducting the pharmacokinetic experiment and Anu Mahadevan (Organix, Inc.) for providing samples of O-1812 and O-1839. This work was supported by National Institutes of Health, National Institute on Drug Abuse Grant DA-016644 and Training Grant DA-07027. Synthesis of O-1812 and O-1839 was supported by NIH/NIDA grant DA-09789 to Anu Mahadevan.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Artmann A, Petersen G, Hellgren LI, Boberg J, Skonberg C, Nellemann C, Hansen SH, Hansen HS. Influence of dietary fatty acids on endocannabinoid and N-acylethanolamine levels in rat brain, liver and small intestine. Biochim Biophys Acta. 2008;781:200–212. doi: 10.1016/j.bbalip.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Berger A, Crozier G, Bisogno T, Cavaliere P, Innis S, Di Marzo V. Anandamide and diet: inclusion of dietary arachidonate and docosahexaenoate leads to increased brain levels of the corresponding N-acylethanolamines in piglets. Proc Natl Acad Sci USA. 2001;98:6402–6406. doi: 10.1073/pnas.101119098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray GA. Use and abuse of appetite-suppressant drugs in the treatment of obesity. Ann Intern Med. 1993;119:707–713. doi: 10.7326/0003-4819-119-7_part_2-199310011-00016. [DOI] [PubMed] [Google Scholar]
- Burston JJ, Wiley JL, Craig AA, Selley DE, Sim-Selley LJ. Regional enhancement of CB1 receptor desensitization in female adolescent rats following repeated Δ9-tetrahydrocannabinol exposure. Bri J Pharmacol. 2010;161:103–112. doi: 10.1111/j.1476-5381.2010.00870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butera PC. Estradiol and the control of food intake. Physiol Behav. 2010;99:175–80. doi: 10.1016/j.physbeh.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll ME, Lynch WJ, Roth ME, Morgan AD, Cosgrove KP. Sex and estrogen influence drug abuse. Tr Pharmacol Sci. 2004;25:273–279. doi: 10.1016/j.tips.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Carta M, Fadda F, Stancampiano R. Tryptophan-deficient diet increases the neurochemical and behavioral response to amphetamine. Brain Res. 2006;1094:86–91. doi: 10.1016/j.brainres.2006.03.118. [DOI] [PubMed] [Google Scholar]
- Childs CE, Romeu-Nadal M, Burdge GC, Calder PC. The polyunsaturated fatty acid composition of hepatic and plasma lipids differ by both sex and dietary fat intake in rats. J Nutr. 2010;140:245–250. doi: 10.3945/jn.109.115691. [DOI] [PubMed] [Google Scholar]
- Cottone P, Sabino V, Roberto M, Bajo M, Pockros L, Frihauf JB, Fekete EM, Steardo L, Rice KC, Grigoriadis DE, Conti B, Koob GF, Zorrilla EP. CRF system recruitment mediates dark side of compulsive eating. Proc Natl Acad Sci USA. 2009;106:20016–20020. doi: 10.1073/pnas.0908789106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kloet AD, Woods SC. Minireview: Endocannabinoids and their receptors as targets for obesity therapy. Endocrinology. 2009;150:2531–2536. doi: 10.1210/en.2009-0046. [DOI] [PubMed] [Google Scholar]
- Deas D, Brown ES. Adolescent substance abuse and psychiatric comorbidities. J Clin Psychiatry. 2006;67:e02. doi: 10.4088/jcp.0706e02. [DOI] [PubMed] [Google Scholar]
- Fattore L, Spano MS, Altea S, Angius F, Fadda P, Fratta W. Cannabinoid self-administration in rats: sex differences and the influence of ovarian function. Br J Pharmacol. 2007;152:795–804. doi: 10.1038/sj.bjp.0707465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fergusson DM, Horwood LJ, Lynskey MT, Madden PA. Early reactions to cannabis predict later dependence. Arch Gen Psychiatry. 2003;60:1033–1039. doi: 10.1001/archpsyc.60.10.1033. [DOI] [PubMed] [Google Scholar]
- Foltz RL, McGinnis KM, Chinn DM. Quantitative measurement of Deta-9-tetrahydrocannabinol and two major metabolites in physiological specimens using capillary column gas chromatography negative ion chemical ionization mass spectrometry. Biomed Mass Spectrom. 1983;10:316–323. doi: 10.1002/bms.1200100503. [DOI] [PubMed] [Google Scholar]
- Harrold JA, Elliott JC, King PJ, Widdowson PS, Williams G. Down-regulation of cannabinoid-1 (CB-1) receptors in specific extrahypothalamic regions of rats with dietary obesity: a role for endogenous cannabinoids in driving appetite for palatable food? Brain Res. 2002;952:232–238. doi: 10.1016/s0006-8993(02)03245-6. [DOI] [PubMed] [Google Scholar]
- Koob GF. Stress, corticotropin-releasing factor, and drug addiction. Ann N Y Acad Sci. 1999;897:27–45. doi: 10.1111/j.1749-6632.1999.tb07876.x. [DOI] [PubMed] [Google Scholar]
- Kramer JR, Chan G, Dick DM, Kuperman S, Bucholz KK, Edenberg HJ, Polgreen LA, Hesselbrock VM, Schuckit MA, Nurnberger JI, Kapp ES, Porjesz B, Bierut LJ. Multiple-domain predictors of problematic alcohol use in young adulthood. J Stud Alcohol Drugs. 2008;69:649–659. doi: 10.15288/jsad.2008.69.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law MY, Slawson MH, Moody DE. Selective involvement of cytochrome P450 2D subfamily in in vivo 4-hydroxylation of amphetamine in rat. Drug Metab Dispos. 2000;28:348–353. [PubMed] [Google Scholar]
- Loebens M, Barros HM. Diet influences cocaine withdrawal behaviors in the forced swimming test. Pharmacol Biochem Behav. 2003;74:259–267. doi: 10.1016/s0091-3057(02)00924-3. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Roth ME, Carroll ME. Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacology (Berl) 2002;164:121–137. doi: 10.1007/s00213-002-1183-2. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Coddington SB, Karelitz JL, Jetton C, Scott JA, Wilson AS, Lerman C. Variability in initial nicotine sensitivity due to sex, history of other drug use, and parental smoking. Drug Alcohol Depend. 2009;99:47–57. doi: 10.1016/j.drugalcdep.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisetsky EM, Chao YM, Dierker LC, May AM, Striegel-Moore RH. Disordered eating and substance use in high-school students: results from the Youth Risk Behavior Surveillance System. Int J Eat Disord. 2008;41:464–470. doi: 10.1002/eat.20520. [DOI] [PubMed] [Google Scholar]
- Priego T, Sanchez J, Pico C, Palou A. Sex-associated differences in the leptin and ghrelin systems related with the induction of hyperphagia under high-fat diet exposure in rats. Horm Behav. 2009;55:33–40. doi: 10.1016/j.yhbeh.2008.07.010. [DOI] [PubMed] [Google Scholar]
- Rodriguez de Fonseca F, Cebeira M, Ramos JA, Martin M, Fernandez-Ruiz JJ. Cannabinoid receptors in rat brain areas: sexual differences, fluctuations during estrous cycle and changes after gonadectomy and sex steroid replacement. Life Sci. 1994;54:159–170. doi: 10.1016/0024-3205(94)00585-0. [DOI] [PubMed] [Google Scholar]
- Roth ME, Cosgrove KP, Carroll ME. Sex differences in the vulnerability to drug abuse: a review of preclinical studies. Neurosci Biobehav Rev. 2004;28:533–546. doi: 10.1016/j.neubiorev.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Sevak RJ, Koek W, Owens WA, Galli A, Daws LC, France CP. Feeding conditions differentially affect the neurochemical and behavioral effects of dopaminergic drugs in male rats. Eur J Pharmacol. 2008;592:109–115. doi: 10.1016/j.ejphar.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim-Selley LJ. Regulation of cannabinoid CB1 receptors in the central nervous system by chronic cannabinoids. Crit Rev Neurobiol. 2003;15:91–119. doi: 10.1615/critrevneurobiol.v15.i2.10. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Thakur GA, Nikas SP, Makriyannis A. CB1 cannabinoid receptor ligands. Mini Rev Med Chem. 2005;5:631–640. doi: 10.2174/1389557054368772. [DOI] [PubMed] [Google Scholar]
- Tseng AH, Harding JW, Craft RM. Pharmacokinetic factors in sex differences in Delta-9-tetrahydrocannabinol-induced behavioral effects in rats. Behav Brain Res. 2004;154:77–83. doi: 10.1016/j.bbr.2004.01.029. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Baler R, Telang F. Imaging dopamine’s role in drug abuse and addiction. Neuropharmacology. 2009;56(Suppl 1):3–8. doi: 10.1016/j.neuropharm.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S, Doshi M, Hamazaki T. n-3 Polyunsaturated fatty acid (PUFA) deficiency elevates and n-3 PUFA enrichment reduces brain 2-arachidonoylglycerol level in mice. Prostaglandins Leukot Essent Fatty Acids. 2003;69:51–59. doi: 10.1016/s0952-3278(03)00056-5. [DOI] [PubMed] [Google Scholar]
- Wellman PJ, Nation JR, Davis KW. Impairment of acquisition of cocaine self-administration in rats maintained on a high-fat diet. Pharmacol Biochem Behav. 2007;88:89–93. doi: 10.1016/j.pbb.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley JL, Martin BR. Cannabinoid pharmacology: implications for additional cannabinoid receptor subtypes. Chem Phys Lipids. 2002;121:57–63. doi: 10.1016/s0009-3084(02)00146-9. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Smith FL, Razdan RK, Dewey WL. Task specificity of cross-tolerance between Delta-9-tetrahydrocannabinol and anandamide analogs in mice. Eur J Pharmacol. 2005;510:59–68. doi: 10.1016/j.ejphar.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Woolridge E, Barton S, Samuel J, Osorio J, Dougherty A, Holdcroft A. Cannabis use in HIV for pain and other medical symptoms. J Pain Symptom Manage. 2005;29:358–367. doi: 10.1016/j.jpainsymman.2004.07.011. [DOI] [PubMed] [Google Scholar]
- Yamamoto I, Watanabe K, Narimatsu S, Yoshimura H. Recent advances in the metabolism of cannabinoids. Int J Biochem Cell Biol. 1995;27:741–746. doi: 10.1016/1357-2725(95)00043-o. [DOI] [PubMed] [Google Scholar]