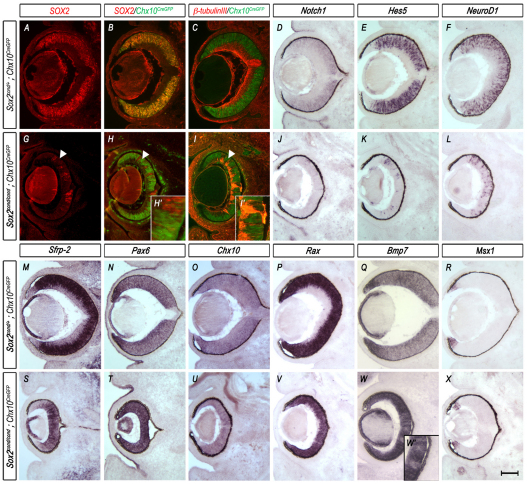
Fig. 5.
Mosaic ablation of SOX2 in neural progenitor cells using Chx10CreGFP results in loss of neuronal differentiation, maintenance of optic cup progenitor genes and central expansion of the optic cup margin. (A-X) Horizontal sections through the eyes of E14.5 control (Sox2cond/+; Chx10CreGFP) and mutant (Sox2cond/cond; Chx10CreGFP) mouse embryos. (A-C,G-I′) Chx10CreGFP reporter (B,C,H-I′; green) double labeled with SOX2 (A,B,G-H′; red) or β-tubulin III (C,I,I′; red) shows expression throughout the whole optic cup in control eyes. In mutant eyes, CRE-GFP is mutually exclusive of SOX2 (H) and β-tubulin III (I). Arrowheads indicate regions in which SOX2 has been ablated. (D-F,J-X) In situ hybridization (ISH) of Notch1 (D,J), Hes5 (E,K), NeuroD1 (F,L), Sfrp2 (M,S) and the optic cup progenitor genes Pax6 (N,T), Chx10 (O,U) and Rax (P,V) shows loss of expression of members of the Notch1 signaling pathway (J-L), central restriction of the Wnt antagonist Sfrp2 (S), upregulation of Pax6 (T) and maintenance of the progenitor markers Chx10 (U) and Rax (V) in regions where SOX2 has been ablated (G) when compared with wild-type controls (D-F,M-P). ISH of the CE marker Bmp7 (Q,W,W′) shows expansion into the prospective NR of mutant embryos, whereas Msx1 (R,X) shows little difference in expression between the control and mutant eye. Scale bar: 200 μm.