Figure 1.
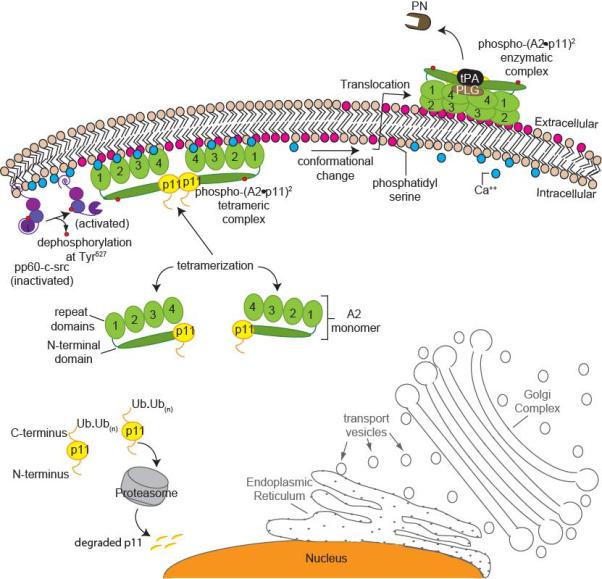
A stress-related working model for heterotetrameric (A2•p11)2 complex formation and translocation to the cell surface. (A2•p11)2 cell surface translocation and activation is independent of the endoplasmic reticulum (ER)-Golgi pathway (outlined in grey). Unpartnered p11 monomers (yellow) within the cell are polyubiquitinated and degraded by the proteasome (grey). Annexin A2 (A2) monomers (green) are bound to p11 subunits. Under stress, intracellular calcium levels increase in response to various stimuli (blue), and this increases the affinity of the (A2•p11)2 complex for binding to anionic phospholipid (shown in dark pink) at the inner membrane surface. There, pp60-c-src kinase (purple) becomes activated following dephosphorylation of Tyr527 within its kinase domain, while Tyr416 within its SH2 domain remains phosphorylated. Once the (A2•p11)2 complex is phosphorylated (red circles) by pp60-c-src, it becomes more tightly associated with inner leaflet phosphatidyl serine (Ptd-L-Ser). The newly phospho-(A2•p11)2 complex may undergo a conformational change, making it more susceptible to translocation to the outer membrane surface. On the outer membrane surface, phospho-(A2•p11)2 associates with plasminogen (PLG) and tissue plasminogen activator (tPA), giving rise to active plasmin (PN).
