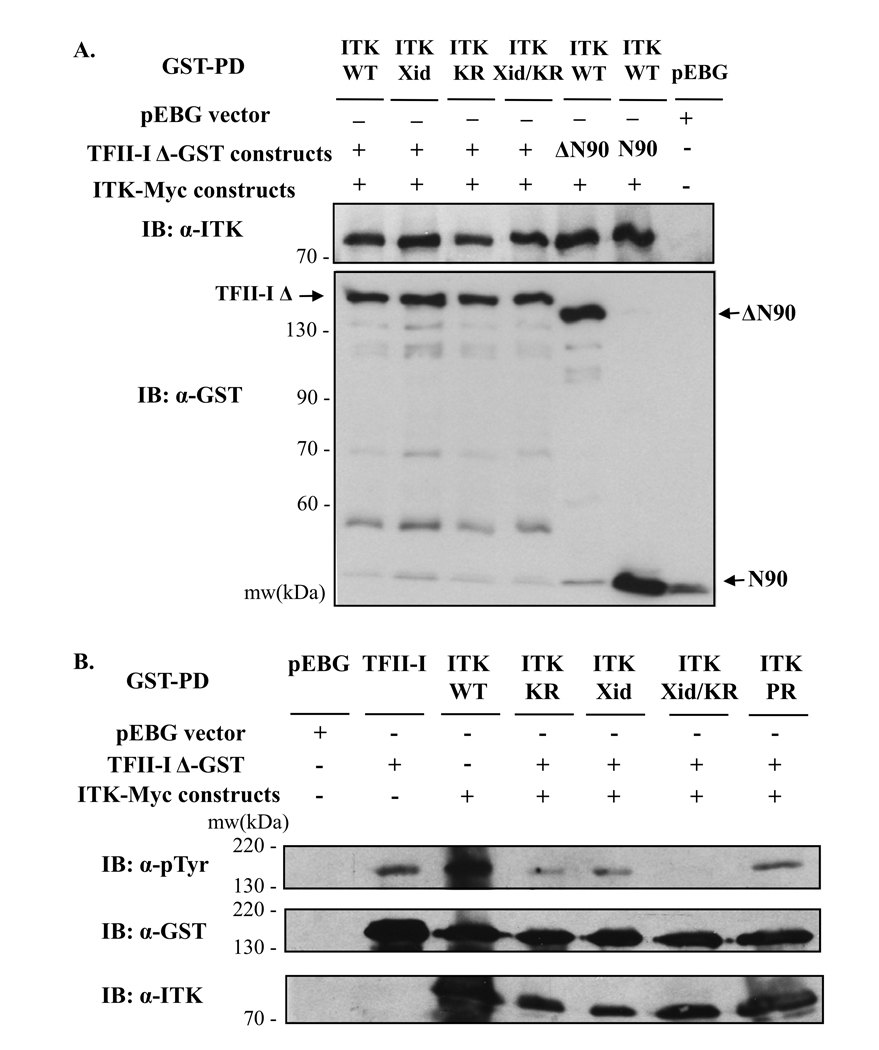
Figure 5. Characterization of ITK-TFII-I domain interactions and TFII-I tyrosine phosphorylation.
(A, B) GST-tagged wild type or mutant (ΔN90, N90) TFII-I-Δ constructs were expressed in the presence or absence of Myc-tagged wild type (WT) or mutant (kinase-dead K390R (KR), R29C (Xid) or the P158A, P159A double mutation (PR)) Itk in COS-7 cells and subjected to whole cell extract GST pull-down assays (GST-PD). The presence or absence of TFII-I-GST and/or ITK-Myc vectors is indicated by (+, ΔN90, N90) or (−) respectively. The control pEBG vector was used as a negative control. (A) The presence of Itk in the GST-PD complexes was detected by immunoblotting (IB) using anti-Itk Ab (upper panel). The membrane was stripped and re-probed with anti-GST Ab to reveal TFII-I-GST protein levels (lower panel). Fainter bands recognized by the anti-GST Ab at lower molecular weights than TFII-I represent protein degradation products, or co-migration with the gel running front (bottom). (B) Following GST-PD, TFII-I-Δ phosphotyrosine levels were assessed by immunoblotting with anti-p-Tyr Ab (upper panel). The presence of Itk in the GST-PD complexes was detected by immunoblotting (IB) using anti-Itk Ab (bottom panel). The membrane was stripped and re-probed with anti-GST Ab to reveal TFII-I-GST protein levels (middle panel). Data are representative of at least two experiments for (A) and (B).