Abstract
Background
Prognostication is a core component of palliative care consultation. We sought to incorporate predicted survival into the routine practice of our hospital-based palliative care team.
Methods
The predicted survival was determined by the physician and/or nurse at the time of initial palliative care consultation using categories that parallel the rough time frames often shared with patients and used in planning care: (1) ≤3 days, (2) 4 days to 1 month, (3) >1 month to 6 months, (4) >6 months. One year later, survival status at 6 months was determined using death certificates, the Social Security online database, and other methods.
Results
Over 1 year, complete data were obtained for 429 of 450 (95.3%) consecutive new patient consults. Patients' mean and median age was 63, 48.5% had cancer, 83% were Caucasian, and 50% were female. For the 283 patients who were discharged alive, median survival was 18 days and 58 patients were still alive after 6 months. Fifty-eight percent of patients were assigned to the correct survival category, whereas 27% of prognoses were too optimistic and 16% were too pessimistic. In logistic regression analysis, predicted survivals of ≤3 days were much more likely to be accurate than longer predictions.
Discussion
The team recorded a predicted survival in 95% of new patient consults. Fifty-eight percent accuracy is in line with prior literature. Routinely incorporating survival prediction into palliative care consultation raised a number of questions. What decisions were made based on the 42% incorrect prognoses? Did these decisions negatively affect care? Survival prediction accuracy has potential as a quality measure for hospital-based palliative care programs, however to be truly useful it needs to be shown to be “improveable” and the downstream effects of predictions need to be better understood.
Introduction
Prognostication is a core component of hospital-based palliative care consultation.1 Predicting survival, in particular, is important as it may prove the deciding factor in decisions to pursue aggressive treatments,2 to change goals of care, or decisions to leave the hospital.3 Patients with limited predicted survival, in particular, may choose to forego disease oriented treatments and to seek care in settings other than acute care hospitals.
Predicted survival (PS) has been studied in many different settings, most commonly in advanced cancer, and generally found to have discriminatory ability and to be highly correlated with actual survival (AS) but to be miscalibrated (and more commonly overoptimistic).4,5 While many studies have examined clinicians' survival predictions and tools for estimating prognosis based on clinical findings and laboratory studies it is not yet clear how to apply these findings in clinical care. After an exhaustive review of the advanced cancer literature, a Working Group of the Research Network of the European Association for Palliative Care concluded: “There is no study on prognostic factors aimed at evaluating whether an accurate prediction of survival can improve actual clinical care; that is, there is no impact study concerning the role of prognostic tools in improving decision making in the palliative care of advanced cancer.” Nevertheless, the working group did recommend that clinicians factor life expectancy into treatment decisions for patients with advanced cancer. Thus there is a translational gap between the “basic science” of survival prediction and its application in clinical practice. For nonmetastatic cancers and diseases other than cancer the data on clinician prediction of survival, prognostic tools, and how to use them are even less well developed.
This article addresses these gaps, describing our incorporation of routine clinician survival prediction into our processes of care for every patient seen by a hospital-based palliative care service, including patients with and without cancer and patients who are not thought to be terminally ill.
Context
The Palliative Medicine and Comfort Care Team (PMCCT) at Oregon Health & Science University (OHSU) serves adult patients and families with any diagnosis in a tertiary academic medical center. In a previous study we found that approximately 2 of 3 of patients were discharged alive, but that their median survival after discharge was only 12 days, with 25% dying within 72 hours.6 Not coincidentally, the most common reasons for consulting the service were to clarify prognosis and goals of care and to assist with discharge planning. Clarifying prognosis can take multiple forms and includes functional and quality of life outcomes, not just predicted survival. To better understand predicted survival, the team in general attempts to identify a consensus opinion among treating clinicians. With the treating team's permission and patient or family consent, the PMCCT will share the prognosis with the patient or family, often in a family meeting. Frequently there are disagreements and uncertainty about prognosis and the team does its best to convey this information as well.
Most studies ask clinicians to predict survival in days and compare the PS to the AS4, 5, but such precise estimates are uncommon in clinical practice. Indeed, rather than predicting the number of days a patient has to live, a more common approach when talking to patients and families about predicted survival is to give a rough estimate such as “a few months” or a range such as “days to weeks” or “weeks to months.”7 This approach acknowledges the clinician's lack of precision and encourages patients and families to think in terms of different possible survival time frames. While purposefully vague, these predictions can help patients and families decide whether or not to continue pursuing life prolonging therapy and to plan for care after the hospitalization.
Methods
This study was approved by the OHSU Institutional Review Board. Patients included all new adult inpatients seen by the palliative medicine and comfort care team at Oregon Health and Science University from July 1, 2006 through June 30, 2007. Survival predictions were framed in categorical terms that parallel the rough time frames (e.g., hours to days, days to weeks, etc.) often told to patients and used in planning care. The categories were defined as follows:
Category 1: ≤3 days—Imminently dying patients who are likely to die in the hospital but also meet the criterion for local inpatient hospice care. The two local inpatient hospice houses (at that time) would accept patients who wished to be transferred if physicians believed the patients would die in less than 3 days, even if the patients did not meet other inpatient skilled criteria.
Category 2: 4 to 30 days—These patients were thought likely to die fairly soon but could discharge from the hospital with adequate caregiving support with hospice available if desired.
Category 3: ≥31 days to 180 days—These patients were also thought likely to die but required plans for a longer period of caregiving after hospital discharge with hospice available if desired.
Category 4: >180 days—These patients were thought likely to live more than 6 months and may require longer term caregiving and are not yet eligible for hospice care under the Medicare Hospice Benefit.
Predicted survival was determined by the PMCCT at the initial consult, generally within 24 hours of first patient contact. The consultation and survival prediction processes were not standardized but generally involved a history and physical examination, chart review, discussion of the case with the treating team, and in many cases a patient/family conference. New consults were generally seen by a physician and advance practice nurse simultaneously. In most cases, the PMCCT conferred with the treating team, the patient's primary care physician, and/or the patient's outpatient subspecialty physician about the patient's prognosis when uncertain. After the initial evaluation was complete, one of the PMCCT members recorded his or her survival prediction in an electronic database along with the reason for consultation, the attending physician's name, the diagnostic category, and his or her impression of the patient's goals of care. If the team members felt they could not determine predicted survival, they could mark “uncertain.” Other demographic data were obtained from the hospital administrative database.
Actual survival was calculated as the number of days from the initial consult date until the date of death. Dates of death for patients who died were determined through a series of methods. First, we looked for patients' death certificates in the Oregon Health Divisions records in August 2008, approximately 1 year after consultation and recorded dates of death for patients who had died. For the remaining patients we ascertained whether they were still living more than 180 days after the consult date by querying the online Social Security Death Index. For patients who did not appear in either database we confirmed whether they were alive at 180 days or not by reviewing their electronic medical records, contacting their treating physicians, or contacting their care facility.
Data were analyzed using SPSS (SPSS Inc., Chicago, IL). Tests of differences in categorical data were assessed using the χ2 statistic and tests of mean differences in continuous data were assessed using t tests. In addition to descriptive statistics a direct logistic regression analysis was used to explore the association between five factors available to the palliative care team at the time of consult and the dependent variable, prognostic accuracy. The dependent variable, prognostic accuracy, was created by categorizing cases into those where prognosis for survival was accurate versus cases where prognosis for survival was inaccurate (either optimistic or pessimistic). A categorical independent variable was created for predicted survival using three dummy variables to compare 4–30 days, 31–180 days, and >180 days with the reference group ≤3 days.
Results
The team performed 450 new consults in the 1-year time frame. A predicted survival was recorded for 429 (95%) of these patients, and actual survival was ascertained for all 450. In comparison to the 429, the 21 patients that the team marked “uncertain” did not differ in gender but were, on average, younger (mean age 53 versus 63 years, p = 0.006), less likely to have cancer (19% versus 49%, p = 0.012), had differing goals of care (0% comfort, 38% palliation, and 62% cure versus 27% comfort, 50% palliation, and 23% cure, p < 0.001), and lived longer (mean survival 63 versus 30 days, p = 0.046). The demographic characteristics of the 429 patients (with a predicted survival) further analyzed in this paper are presented in Table 1.
Table 1.
Demographics and Reason for Consult
| N = 429 | ||
|---|---|---|
| % Minority ethnic group | 17% | |
| % Female | 50.1% | |
| Mean age (SD) | 62.7 years (16.4) | |
| Consulting service | Medical intensive care unit | 15.9% |
| Medicine or family practice ward | 29.8% | |
| Hematology/oncology ward | 17.3% | |
| Other | 37% | |
| Diagnostic category | Cancer | 48.5% |
| Neurologic disease | 16.6% | |
| Cardiovascular disease | 8.2% | |
| Liver disease | 6.8% | |
| Multi organ failure | 5.6% | |
| Lung disease | 6.3% | |
| Other | 8% | |
| Reason for consulta | Clarify prognosis/goals of care | 62% |
| Discharge planning | 50.1% | |
| Psychosocial support | 31.7% | |
| Pain/symptom management | 28.9% | |
| Care of imminently dying patient | 24% | |
| Discharge disposition | Died during hospitalization | 34.0% |
| Home, no home health, no hospice | 16.8% | |
| Home with hospice | 16.6% | |
| Inpatient hospice | 12.4% | |
| Skilled nursing facility | 12.4% | |
| Home with home health | 7% | |
| Died in a different hospital | 0.9% |
Percentages add up to >100% because patients could have more than 1 reason for consult.
SD, standard deviation.
Accuracy of predicted survival
The team was able to accurately predict survival in 247 of 429 cases (57.6%), whereas they were overpessimistic (i.e., actual survival was longer than predicted survival) in 67 (15.6%) cases and overoptimistic (i.e., actual survival was shorter than predicted survival) in 115 (26.8%). In all, 409 of 429 (95.3%) predictions were accurate or within plus or minus one category—that is less than 5% of predictions were off by more than one category. Thirty-day readmission rates were not different for patients whose survival was predicted accurately versus inaccurately (3.2% versus 5.5%, χ2 = 1.33, p = 0.33) however overall readmission rate was very low.
For 125 patients predicted to die in 0–3 days, 107 (85.6%) died in the predicted 3-day interval with most of these dying on the day of consult (50/125; 40%) or the following day (38/125; 30.4%). Ten of 125 patients (8%) lived longer than a week, with 1 patient living 147 days. At the time of initial consult, this patient with advanced congestive heart failure was septic with falling blood pressure despite maximal vasopressor therapy, and following a family conference there had been a decision to pursue comfort measures only; however, despite withdrawal of antibiotics and vasopressors his hypotension and sepsis resolved and he was able to be discharged to a skilled nursing facility within a week. Ninety-eight (78.4%) of patients in this category died in the hospital.
For 134 patients predicted to survive 4–30 days, 75 (54%) died in the predicted time frame, with 42 (30.2%) dying within the first 3 days, 17 (12.2%) lived 31–180 days, and 5 (3.6%) lived more than 180 days. Thirty-eight (27.3%) of the patients in this category died in the hospital.
For 132 patients predicted to survive 31–180 days, 46 (34.8%) died in the predicted time frame, with 9 (6.8%) dying in 0–3 days, 50 (37.9%) dying in 4–30 days, and 27 (20.5%) living more than 180 days. 9 (6.8%) of the patients in this category died in the hospital.
For 33 patients predicted to survive more than 180 days, 19 (57.6%) were alive at 180 days, whereas 1 (3%) died in 3 days, 3 (9.1%) died in 4–30 days, and 10 (30.3%) died in 31–180 days. The patient who died in 3 days was not initially felt to be terminally ill after being admitted for dehydration and rhabdomyolysis following a fall in her home. The PMCTT was consulted because she said she was saying she wanted to “be left alone” but deemed her not terminally ill because she did not have a terminal diagnosis and had been living independently up to the time of her fall. However, the following day she asserted that she wanted comfort measures only, declined food and fluids, and probably died of a pulmonary embolism 3 days after consultation. She was the only patient in this category who died in the hospital.
Comparing the 32 patients who lived more than 180 days (but were predicted to die sooner) to all other patients, they were similar in age (66.2 versus 62.5 years, p = .223), somewhat more likely to be women (65.6% versus 48.9%, p = 0.096), more likely to have cardiovascular or pulmonary disease (37.6% versus 12.6%, p = 0.015) than cancer or neurologic disease (40.7% versus 67.2%, p = 0.015), and much less likely to have comfort as their primary goal (0% versus 27.7%, p < 0.001). Of note the PMCCT was asked to help clarify prognosis and/or goals of care for 28 of these 32 patients.
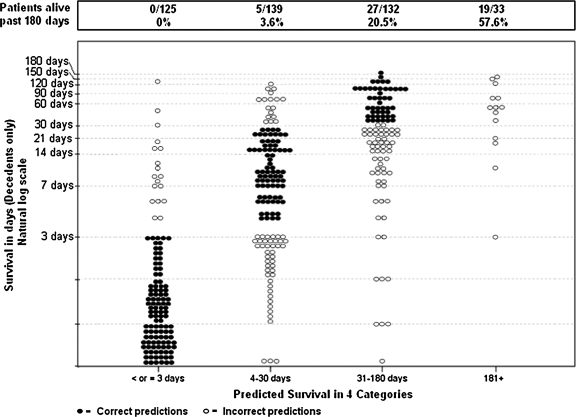
These data are presented in full detail in Table 2 and Figure 1. Table 2 compares predicted survival categories to actual survival divided into the same categories. Light gray shading has been added to predictions that were off by one category, dark grey shading for predictions off by two categories, and black shading for predictions (only one case) off by three categories. Figure 1 divides patients into the four predicted survival categories and shows their actual survival on a natural log scale. Correct predictions are shown as black dots, incorrect predictions as open circles. The figure visually illustrates how “close” or “far off” predictions were.
Table 2.
Predicted Survival Category vs. Actual Survival Category
| |
|
|
Predicted survival |
Total |
|||
|---|---|---|---|---|---|---|---|
| ≤ or 3 days | 4–30 days | 31–180 days | 181+ | ≤ or 3 days | |||
| Actual Survival | 0–3 days | n | 107 | 42 | 9 | 1 | 159 |
| % | 85.6% | 30.2% | 6.8% | 3.0% | 37.1% | ||
| 4–30 days | n | 16 | 75 | 50 | 3 | 144 | |
| % | 12.8% | 54.0% | 37.9% | 9.1% | 33.6% | ||
| 31–180 days | n | 2 | 17 | 46 | 10 | 75 | |
| % | 1.6% | 12.2% | 34.8% | 30.3% | 17.5% | ||
| 181 or more days | n | 0 | 5 | 27 | 19 | 51 | |
| % | .0% | 3.6% | 20.5% | 57.6% | 11.9% | ||
| Total | n | 125 | 139 | 132 | 33 | 429 | |
| % | 100.0% | 100.0% | 100.0% | 100.0% | 100.0% | ||
Note: Light gray shading indicates predictions off by one category compared to actual survival, dark gray shading for predictions off by two categories, and black shading for predictions off by three categories.
FIG. 1.
Predicted survival category versus actual survival in days (natural log scale).
What was associated with accurate predictions?
A direct logistic regression analysis was performed on the binary outcome variable, prognostic accuracy using five predictor variables: patient age, cancer diagnosis, duration of hospital stay prior to consultation, whether a consult was requested to clarify prognosis/goals of care, and predicted survival. A test of the full model with all predictors against a constant-only model was statistically significant, χ2 (7, N = 429) = 81.66, p < 0.001), indicating that the predictors, as a set, reliably distinguished between accurate and inaccurate prognosis. The full model was slightly better at classifying cases where the team made an accurate prediction (74% correctly predicted) than cases where the team made an inaccurate prediction (62% correctly predicted) for an overall success rate of just 69%.
Table 3 shows the odds ratios, associated 95% confidence intervals, and p values for each of the predictor variables. Controlling for all other variables in the model, predicted survival of ≤3 days remained much more likely to be accurate than longer predicted survivals. Compared with predictions of ≤3 days, predictions of 4–30 days had 80% lower odds of being accurate, predictions of 31–180 days had 91% lower odds of being accurate, and predictions of >180 days had 78% lower odds of being accurate.
Table 3.
Logistic Regression Analysis of Prognostic Accuracy (N = 429) To Determine Factors that Predict Prognostic Accuracy
| Predictor | Odds ratio (95% CI) | p value |
|---|---|---|
| Patient age | 0.99 (0.97–1.0) | 0.05 |
| Cancer diagnosis | 1.25 (0.80–1.96) | 0.32 |
| Duration of hospital stay prior to consult | 1.0 (0.98–1.01) | 0.32 |
| Consult requested to clarify goals | 0.80 (0.50–1.26) | 0.33 |
| Survival Prediction ≤3 vs. 4–30 days | 0.20 (0.10–0.37) | 0.001 |
| Survival Prediction ≤3 vs. 31–180 days | 0.09 (0.05–0.17) | 0.001 |
| Survival Prediction ≤3 vs. >180 days | 0.22 (0.09–0.53) | 0.001 |
CI, confidence interval.
Did overestimation or underestimation of prognosis lead to suboptimal discharge plans? For example, were patients not referred to hospice care because their prognosis was overestimated? Such conclusions would have required a case-by-case analysis that was not done in this study; however, in our sample patients discharged home without hospice had approximately the same frequency of overoptimistic predictions (24/72; 33%) as patients discharged home with hospice (23/71; 32.4%).
Discussion
We found that we could incorporate routine prediction of survival into the palliative care consultation process and that clinicians provided a prediction in 95% of cases. Survival prediction accuracy was inversely related to survival up to 6 months with accuracy declining from 85.6% to 54% to 34.8% for patients surviving ≤3 days, 4-30 days, and 31-180 days respectively. The logistic regression model confirmed that when the team predicted 0-3 days, they were more likely to be accurate. Greater predictive accuracy in patients closer to dying has been called the “horizon effect” and has not been studied extensively, but data suggest that because clinicians in general tend to be overoptimistic, the longer the predicted survival and the shorter the observed survival, the less accurate predictions will tend to be.4
The overall average accuracy of these predictions, 58%, is in line with prior studies of clinician prediction of survival. For example, a meta-analysis of eight studies comparing predicted and actual survival in patients with advanced cancer, which found that clinician predictions were correct to within 1 week in 25% of cases, correct to within 2 weeks in 43%, and correct to within 4 weeks in 61%.5 Similar to our previous study, 65% of patients were discharged alive although their median survival was 18 days compared with the previous finding of 12 days.6
Allowing clinicians to predict survival within broad ranges may have contributed to their willingness to make predictions, although interestingly it did not dramatically improve their ability to be correct. In presenting these results to the participating clinicians, they wanted to know how “close” they were in the 42% of inaccurate cases. Figure 1 was useful in helping them to see to how far off individual predictions were.
This study raises a number of questions that cannot be answered by the available data. First and foremost, what impact do incorrect predictions have on patients and families? Were decisions made based on the 42% incorrect prognoses that would have been made differently with accurate information? Did these decisions negatively affect care? It may be that the 37% of predictions that were within plus or minus 1 category were still helpful if they were conveyed in the context of an open and honest discussion about goals of care8 and if patients' or family members' predictions were even more overoptimistic or overpessimistic. It is worth noting that the proportion of deaths in hospital decreased in proportion to longer predicted survival: 78.4% for ≤3 days, 27.3% for 4–30 days, 6.8% for 31–180 days, and 3.3% for ≥181 days. While this trend might indicate that care and discharge plans were incorporating survival prediction and thus allowing patients with less dire prognoses to not die in the hospital, much work needs to be done to measure or even understand the connection between survival predictions and clinical care.
Another question raised by routinely incorporating prognosis into palliative care consultation is can health professionals actively improve their abilities to prognosticate—either by getting practice feedback or else by using a standardized tool such as the palliative performance scale?9 Presumably, finding out that they were 58% accurate 2 years after the fact will not improve our team's ability to prognosticate, but would getting this feedback in a timely fashion be useful? It can be very challenging to reliably and promptly know when patients die and the circumstances, particularly for tertiary referral centers caring for patients across large geographic regions. It is also likely that additional clinical details would be needed to understand why a particular prediction may have been accurate or inaccurate.
Regarding using standardized tools to improve clinicians' ability to predict survival, a single study has addressed this question in a study of patients with cancer admitted to a palliative care unit in Japan. Morita et al.10 found that clinician predictions of survival were more accurate (predicting death within ± 28 days) after they started routinely calculating each patient's palliative prognostic index (PPI) score (58% accurate before using the PPI, 77% accurate after, p < 0.01). The PPI was developed for patients with cancer and it is not known whether it would be similarly helpful for noncancer patients. The palliative performance scale has been shown to correlate with patient survival in hospital based palliative care patients with cancer and other diagnoses,11,12 however, it has not been studied to see whether clinicians using the palliative performance scale make more accurate survival predictions. Lau et al.13 have conducted a helpful review of prognostic tools for estimating survival in palliative care that evaluates the strengths, limitations, and research for 11 different tools.
Our study has important limitations. First, it represents the work of a single hospital-based palliative care team which may not be representative of other teams or other palliative care patient populations. Judging predictive accuracy based on an initial assessment does not accurately reflect prognosis in clinical care, which is not fixed at the first encounter but like any clinical opinion, evolves as the patient's clinical course evolves and new data are available. Also, we did not use a standardized process for survival prediction making it difficult to understand the factors contributing to accuracy/error. One question along these lines is whether creating a more formal process to involve multiple team members in generating the prognosis might have improved our accuracy. A study in patients with cancer referred for palliative radiotherapy found that an experienced clinician with 10 years experience was not more accurate than were interns or a multidisciplinary tumor board of 5–10 clinicians (55% versus 61% and 63% predictions correct, respectively).14 Finally, the data we present are insufficient to determine what impact accurate or inaccurate prognostic data have on patients and families.
A final question raised by this study is whether survival prediction accuracy has potential as a quality measure for palliative care. Our results illustrate that hospital-based palliative care teams can incorporate routine survival prediction into their consultative process. The National Consensus Project includes assessing and documenting prognosis and patient/family understanding of prognosis as part of their very first Guideline for Quality Palliative Care15 (Guideline 1.1 “The timely plan of care is based on a comprehensive interdisciplinary assessment of the patient and family”). On the positive side it is relatively easily measurable and with dates of death available on-line through the social security death index (See for example http://ssdi.rootsweb.ancestry.com/) fairly easy to determine. In order to be a useful measure of quality, however, the ability to predict should also be clearly “improveable.” So far, only the Morita study provides any evidence that clinicians can improve their accuracy.10
As the field of hospice and palliative medicine continues to evolve and our understanding of quality palliative care deepens, we hope that future studies will help bridge the translational gap between the science of prognostication and its application in routine care. In particular it would be useful to understand how to make better predictions and to elucidate the link between predictions, communication, decision making, and outcomes.
Acknowledgments
Dr. Fromme is supported by a Career Development Award from the National Cancer Institute K07CA109511 and was supported by the Oregon Clinical and Translational Research Institute (OCTRI), grant number UL1 RR024140 from the National Center for Research Resources (NCRR).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Weissman D. Consultation in palliative medicine. Arch Intern Med. 1997;157:733–737. [PubMed] [Google Scholar]
- 2.Weeks JC. Cook EF. O'Day SJ. Peterson LM. Wenger N. Reding D. Harrell FE. Kussin P. Dawson NV. Connors AF., Jr Lynn J. Phillips RS. Relationship between cancer patients' predictions of prognosis and their treatment preferences. JAMA. 1998;279(21):1709–1714. doi: 10.1001/jama.279.21.1709. [DOI] [PubMed] [Google Scholar]
- 3.Glare P. Sinclair C. Palliative Medicine Review: Prognostication. J Palliat Med. 2008;11(1):84–103. doi: 10.1089/jpm.2008.9992. [DOI] [PubMed] [Google Scholar]
- 4.Glare P. Christakis NA. Predicting survival in patients with advanced disease. In: Doyle D, editor; Hanks G, editor; Cherney N, editor; Calman K, editor. Oxford Textbook of Palliative Medicine. New York: Oxford University Press; 2005. pp. 29–42. [Google Scholar]
- 5.Glare P. Virik K. Jones M. Hudson M. Eychmuller S. Simes J. Christakis N. A systematic review of physicians' survival predictions in terminally ill cancer patients. BMJ. 2003;327:195–198. doi: 10.1136/bmj.327.7408.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fromme E. Bascom P. Smith M. Tolle SW. Hanson L. Hickam DH. Osborne ML. Survival, mortality, and location of death for patients seen by a hospital-based palliative care team. J Palliat Med. 2006;9:903–911. doi: 10.1089/jpm.2006.9.903. [DOI] [PubMed] [Google Scholar]
- 7.Hallenback J. Palliative Care Perspectives. New York: Oxford University Press; 2003. Dying trajectories and prognostication; p. 15. [Google Scholar]
- 8.Finley E. Cassarett D. Making difficult decisions easier: Using prognosis to facilitate transitions to hospice. CA Cance J Clin. 2009;59:250–263. doi: 10.3322/caac.20022. [DOI] [PubMed] [Google Scholar]
- 9.Anderson F. Downing G. Hill J. Palliative Performance Scale (PPS): A new tool. J Palliat Care. 1996;12:5–11. [PubMed] [Google Scholar]
- 10.Morita T. Tsunoda J. Inoue S. Chihara S. Improved accuracy of physicians' survival prediction for terminally ill cancer patients using the Palliative Prognostic Index. Palliat Med. 2001;15:419–424. doi: 10.1191/026921601680419474. [DOI] [PubMed] [Google Scholar]
- 11.Lau F. Maida V. Downing M. Lesperance M. Karlson N. Kuziemsky C. Use of the Palliative Performance Scale (PPS) for end-of-life prognostication in a palliative medicine consultation service. J Pain Symptom Manage. 2009;37:965–972. doi: 10.1016/j.jpainsymman.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 12.Olajide O. Hanson L. Usher BM. Qaqish BF. Schwartz R. Bernard S. Validation of the palliative performance scale in the acute tertiary care hospital setting. J Palliat Med. 2007;10:111–117. doi: 10.1089/jpm.2006.0125. [DOI] [PubMed] [Google Scholar]
- 13.Lau F. Cloutier-Fisher D. Kuziemsky C. Black F. Downing M. Borycki E. Ho F. A systematic review of prognostic tools for estimating survival time in palliative care. J Palliat Care. 2007;23:93–112. [PubMed] [Google Scholar]
- 14.Gripp S. Moeller S. Bölke E. Schmitt G. Matuschek C. Asgari S. Asgharzadeh F. Roth S. Budach W. Franz M. Willers R. Survival prediction in terminally ill cancer patients by clinical estimates, laboratory tests, and self-rated anxiety and depression. J Clin Oncol. 2007;25:3313–3320. doi: 10.1200/JCO.2006.10.5411. [DOI] [PubMed] [Google Scholar]
- 15.National Consensus Project for Quality Palliative Care. Clinical Practice Guidelines for Quality Palliative Care. 2nd. Pittsburgh, PA: 2009. [Google Scholar]