Fig. 2.
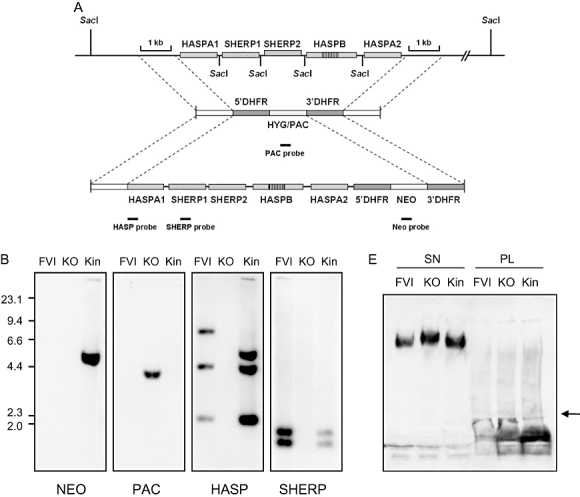
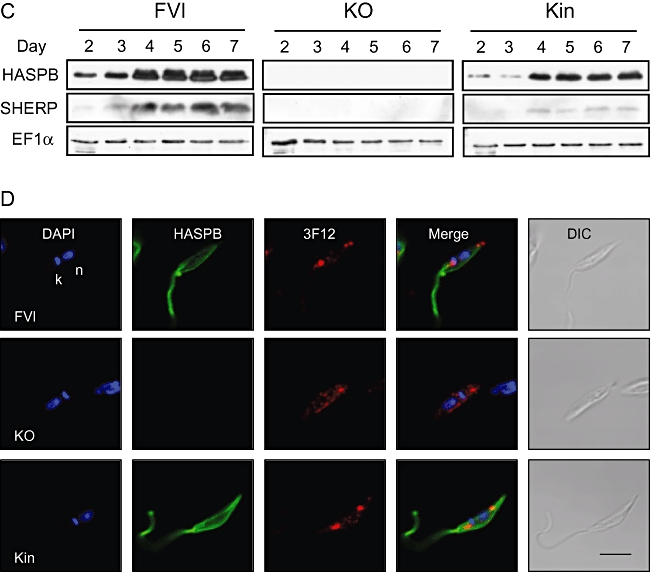
Targeted deletion and complementation of the LmcDNA16 locus.A. Schematic diagram of the LmcDNA16 locus and the plasmid constructs used for deletion and complementation. Flanking sequences used to generate the targeting vectors are shown. The top panel shows the LmcDNA16 locus, which contains two tandem identical copies of the SHERP gene, two non-tandem identical copies of the HASPA gene and a single copy of the HASPB gene. The HASPB open reading frame is highly similar to that of HASPA except for an additional repeat region (McKean et al., 1997a), indicated here by vertical line shading. The second panel shows the plasmid constructs used for targeted deletion of the locus as described previously (McKean et al., 2001) in which the locus was replaced by hygromycin/puromycin resistance genes (HYG/PAC). The third panel shows the plasmid construct used to produce a complemented parasite line in this study. This sequence contains flanking regions sharing identity with two sections of the deletion construct: the 5′ flanking region and the DHFR 3′UTR which is found downstream of the HYG/PAC genes. Correct integration of the complementation construct will replace either HYG or PAC with a single copy of the LmcDNA locus plus a copy of the neomycin resistance gene (NEO). Solid black bars represent fragments used as hybridization probes.B. Southern blot analysis of wild-type and mutant parasite lines. The following lines were analysed: wild type (FVI), HYG/PAC double replacement clone (ΔcDNA16::HYG/ΔcDNA16::PAC, KO) and a complemented double replacement clone in which the PAC gene had been replaced with a single copy of the cDNA16 locus (ΔcDNA16::HYG/ΔcDNA16::PAC/ΔPAC::cDNA16, Kin). Five micrograms of genomic DNA from each parasite line was digested with SacI, size separated through 0.8% agarose, blotted and hybridized with DIG-labelled DNA probes (∼200 bp) as indicated.C. Immunoblotting of wild-type and mutant parasite lines as in (B). Whole-cell lysates taken from parasites grown in culture for 2–7 days were separated by 10% (HASPB, EF1α) or 15% (SHERP) SDS-PAGE and immunoblots probed with polyclonal antisera raised against L. major HASPB and SHERP. A monoclonal antibody recognizing the constitutively expressed protein EF1α (clone CBP-KK1, Millipore) was used to confirm equivalent protein loading. Approximately 1 × 106 parasites were loaded per lane.D. Expression of HASPB and LPG in metacyclic promastigotes of wild-type and mutant parasite lines (described in B and C). Parasites grown in culture for 7 days were agglutinated with peanut lectin and non-agglutinated cells fixed and permeabilized prior to indirect immunofluorescence analysis. DAPI (blue), nuclear and kinetoplast DNA; HASPB (green), 3F12 (red), antibodies specific for metacyclic LPG and HASPB respectively; DIC, differential interference contrast image. Size bar = 5 µm.E. Wild-type and mutant parasite lines (described in B and C) secrete proteophosphoglycans. Pellets prepared by ultracentrifugation of parasite culture supernatants (SN) or whole parasite lysates (PL) were separated by SDS-PAGE and blotted (arrow indicates boundary between 4% stacking gel and 12% resolving gel). The immunoblot was probed with monoclonal antibody LT6 (polyclonal rabbit antiserum against PPG; Ilg et al., 1993).
