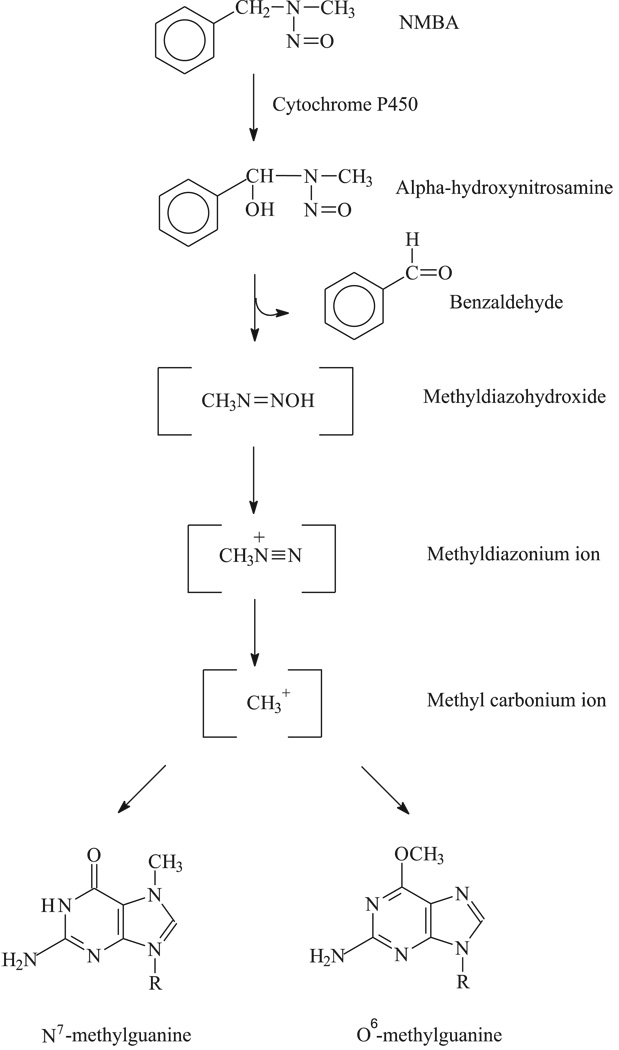
Figure 1.
Hydroxylation pathway of N-nitrosomethylbenzylamine (NMBA) metabolism at the methylene carbon in the rat esophagus. NMBA metabolism involving α-hydroxylation at the methylene carbon atom at the N-nitroso group results in the formation of benzaldehyde and reactive methyl diazonium ions. The reactive intermediate forms both N7 and the highly mutagenic O6 adduct on guanine.