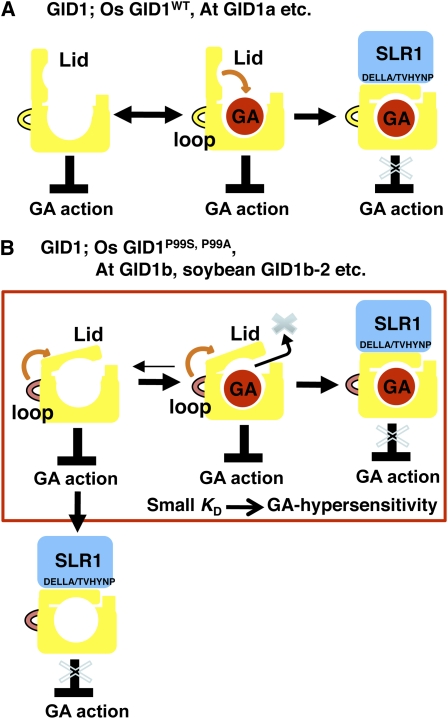
Figure 9.
Molecular Model for Formation of the GA–GID1P99A–SLR1 Complex.
(A) In typical GID1s, such as OsGID1WTand At GID1a, GID1 cannot interact with DELLA protein in the absence of GA. When GA comes into its binding pocket, the lid closes on the binding pocket, which allows the interaction between GID1 and the DELLA/TVHYNP domains of DELLA protein. This interaction between GID1 and DELLA protein leads to degradation of DELLA protein (for instance, SLR1) and allows the derepressed state of GA responses.
(B) In certain GID1s, such as Os GID1P99S and P99A, At GID1b, and soybean GID1b-2 and -3, the lid tends to be closed even in the absence of GA, which promotes the interaction with DELLA protein. The closed state of the GID1P99A lid causes lower accessibility and slower dissociation of GA4. These properties of the receptor lead to GA hypersensitivity in the formation of the GID1–GA–DELLA protein complex. The interactions between GID1 and DELLA protein both with and without GA may allow the derepressed state of GA responses.