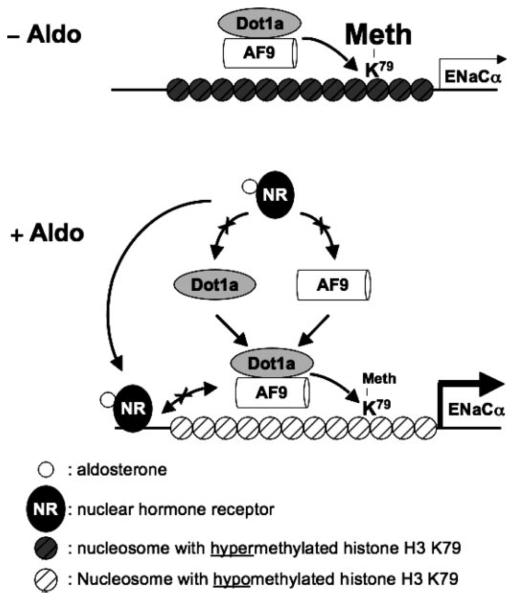
FIGURE 6. Hypothetical model for aldosterone-sensitive repression of ENaCα transcription by Dot1a-AF9 complex modulating histone H3 Lys-79 hypermethylation associated with the ENaCα promoter.
Under basal conditions, Dot1a and AF9 form a nuclear complex that directly or indirectly binds specific sites of the ENaCα promoter, leading to hypermethylation of histone H3 Lys-79 and repression of ENaCα (A). Aldosterone (blank circles) stimulates ENaCα transcription by regulating two different complexes. In the classical pathway, aldosterone binds and activates the nuclear receptors (NR) that are either GR and/or mineralocorticoid receptor homo- or heterodimer to bind GRE for transactivation of ENaCα. In a parallel pathway, aldosterone down-regulates the amount of Dot1a-AF9 complex by reducing Dot1a (15) and AF9 expression, presumably via NR-dependent (shown) or NR-independent mechanisms (not shown) to limit their availability, leading to histone H3 Lys-79 hypomethylation at the specific subregions and release of repression of ENaCα. In either case, AF9-free Dot1a binds DNA nonspecifically and catalyzes histone H3 Lys-79 methylation throughout the genome at the basal level (not shown). The putative counter-interaction between the NR-aldosterone complex and the Dot1a-AF9 complex is also shown and may tune the ultimate level of ENaCα transcription. Similar mechanisms may be applicable to other aldosterone-regulated genes.