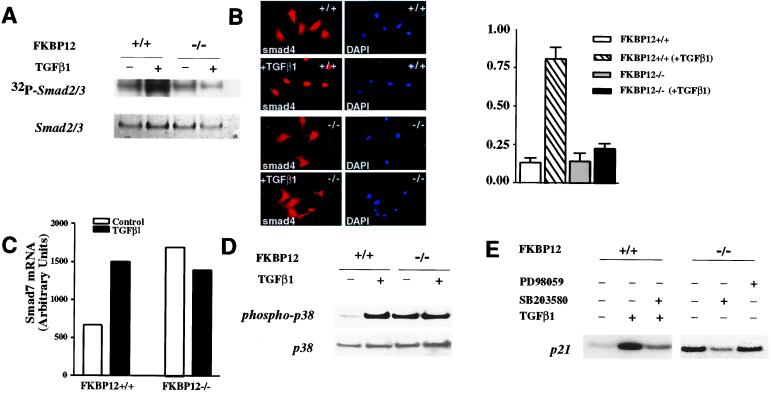
Figure 5.
Overactivation of TGF-β signaling is due to increased p38 MAP kinase signaling. (A) TGF-β1 treatment increases the phosphorylation of SMAD2/3 in FKBP12+/+ but not FKBP12−/− cells. FKBP12+/+ and FKBP12−/− cells were incubated with [32P]orthophosphate. Immunoprecipitation with anti-SMAD2/3 was used to determine the amount of 32P incorporated into SMAD2/3. There was no significant difference in basal SMAD2/3 phosphorylation in the FKBP12+/+ or FKBP12−/− cells. TGF-β1 treatment (30 min) significantly increased SMAD2/3 phosphorylation in FKBP12+/+ but not in FKBP12−/− cells. (B) TGF-β1 treatment increases nuclear localization of SMAD4 in FKBP12+/+ but not in FKBP12−/− cells. After TGF-β1 treatment, nuclear localization of SMAD4 was observed in more than 80% of FKBP12+/+ cells but no significant increase was seen in FKBP12−/− cells. (C) SMAD7 mRNA is significantly higher in FKBP12−/− than FKBP12+/+ cells. TGF-β1 treatment increased SMAD7 mRNA in FKBP12+/+ cells almost to the levels of the FKBP12−/− cells but did not augment the SMAD7 mRNA in the FKBP12−/− cells. (D) p38 MAP kinase is constitutively phosphorylated in FKBP12−/− fibroblasts. TGF-β1 treatment (30 min) increased phosphorylation of p38 in FKBP12+/+ cells; however, p38, in FKBP12−/− cells, was already extensively phosphorylated and TGF-β1 treatment did not increase phosphorylation. No change in total p38 expression was observed relative to actin or total protein. The experiment was replicated three times. (E) Inhibition of p38 MAP kinase blocks p21 induction in FKBP12−/− fibroblasts. SB203580 (20 μM, 24 h), an inhibitor of p38 MAP kinase, blocked the TGF-β1 induction of p21 in FKBP12+/+ cells and reduced p21 in the FKBP12−/− cells. PD98059 (20 μM, 24 h), an inhibitor working upstream of MAP kinase/Erk kinase, did not affect p21 in FKBP12−/− cells.