Abstract
Background:
Hydatid disease is an endemic condition in several parts of the world. Owing to ease of travel, even surgeons in nonendemic areas encounter the disease and should be aware of its optimum treatment. A safe, new method of laparoscopic management of hepatic hydatid disease is described along with a review of the relevant literature.
Methods:
Sixty-six cases of hepatic hydatid disease were operated on laparoscopically using the Palanivelu Hydatid System. The special trocar-cannula system used and the technique of operation are described.
Results:
The majority of the patients presented with pain. Most of the patients had only a single cyst. The right lobe of the liver was most commonly involved. Cysts were bilateral in 4 patients. In 83.3%, simply evacuation of the hydatid cyst by the Palanivelu Hydatid System was done. In 13.7%, this was followed by a left lobectomy, as the cysts were large occupying almost the entire left lobe of the liver. The remnant cavity was dealt with by omentoplasty. The average follow-up period is 5.8 years. There have been no recurrences to date.
Conclusion:
We recommend Palanivelu Hydatid System for management of hepatic hydatid disease. We have found its efficacy to be optimum for preventing spillage, evacuating hydatid cyst contents, performing transcystic fenestration, and for dealing with cyst-biliary communications.
Keywords: Hepatic hydatidosis, Laparoscopy
INTRODUCTION
Hydatidosis is an endemic parasitic disease in Mediterranean countries, North Africa, Turkey, the Middle East, Australia, New Zealand, South America, Baltic areas, the Philippines, Northern China, and the Indian subcontinent. However, physicians and surgeons worldwide may encounter the disease sporadically because of increased travel and immigration.1,2
It is caused by the parasite, Echinococcus granulosus, which is a cestode that lives in the small intestine of dogs and other canines. Eggs are eliminated in the feces and when ingested, liberate their larvae in the duodenum of an intermediate host. The intermediate host can be sheep/ goat (pastoral hydatidosis) or reindeer/moose/caribou (sylvan hydatidosis). Humans are accidental intermediate hosts. The larvae cross the intestinal wall and via the portal system reach the hepatic sinusoids where they develop into cysts. Some larvae are not filtered in the liver, but remain in the blood to reach the next station, the lungs. In addition, some may pass through the pulmonary circulation and travel to other organs. Larva transported in the mesenteric lymphatics are carried to the cisterna chili, the thoracic duct, and into the general circulation, ending up in a variety of distant sites.1,3
The most common site of occurrence of hydatid cysts in humans is the liver (50% to 93%).1,4,5 Left untreated, the cyst grows and follows one of several courses: forms fistulas into adjacent organs or the biliary system, ruptures into the peritoneal cavity causing seeding of multiple daughter cysts throughout the peritoneal cavity, developing daughter cysts within or rarely dying de novo. Older cysts have an increased risk of exogenous daughter cyst formation, which is an important factor for recurrence of disease after surgery.6,7
Different therapies have been suggested. Medical therapy consists of albendazole alone or in combination with praziquantel by which stabilization of the disease has been reported.8–12 A variety of surgical operations have been advocated from complete resection (eg, total pericystectomy or hepatectomy) to minimally invasive procedures (eg, percutaneous aspiration of cysts).6,13–17 The World Health Organization (WHO) recommends percutaneous aspiration, irrigation and re-aspiration (the PAIR approach).18 More recently, reports have been published on laparoscopic surgery for hepatic hydatid cysts.19–25 However, fear of anaphylactic shock resulting from spill-age of hydatid fluid during treatment by the minimally invasive method may be discouraging a wider adoption of this technique.26
This study evaluates the efficacy of a new trocar-cannula system, the Palanivelu Hydatid System (PHS), specifically designed to prevent spillage of hydatid fluid for laparoscopic management of hydatid cysts.
METHODS
In all patients, the diagnosis of echinococcal cyst was based on history, physical examination, ultrasound (US), and computed tomography (CT) scan. Ultrasound was used for follow-up. All patients were treated with albendazole 10 mg/kg/day for at least 2 weeks preoperatively and continued postoperatively for 4 weeks. The exclusion criteria were no informed consent; serious coagulation abnormalities; known allergy to local anesthetics or albendazole; deep-seated cysts; posteriorly located cysts (segments I, VII and VIII); cysts less than 3cm in diameter; pregnancy, or women who refused contraception for the time of albendazole treatment; and cysts characterized by a heterogeneous complex mass (Gharbi type 4) or a calcified wall (Gharbi type 5).
The Palanivelu Hydatid System
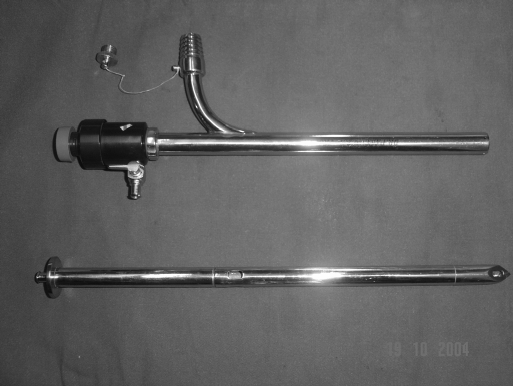
The Palanivelu Hydatid System (PHS) consists of a trocar and cannula along with 5-mm and 3-mm reducers. The trocar is 29-cm long. It is hollow throughout its length to accommodate a suction cannula. Its tip is pyramidal shaped with each facet of the pyramid bearing a fenestration to enable any fluid leaking on its insertion to be sucked into its hollow body by the cannula placed within. Its long shaft also bears 2 fenestrations opposite each other at a distance of 17 cm from the tip. The cannula is 26-cm long with an inner diameter of 12 mm. It has 2 side channels, one for gas insufflation and another for suction. The suction channel has an inner diameter of 10 mm. Its outer nozzle is designed so that the suction tube can be fit onto it in an airtight manner (Figure 1).
Figure 1.
Palanivelu Hydatid System.
Technique
After introducing the camera port through the umbilicus following creation of pneumoperitoneum, the hydatid cyst is identified on the surface of the liver. Then, the PHS trocar with cannula is introduced into the peritoneal cavity directly over the hydatid cyst. Once inside the peritoneal cavity, the trocar is removed and the cannula alone is advanced till its tip is in total contact with the hydatid cyst surface. Suction is applied through the side channel to maintain the contact between the cyst and the cannula opening. Thereafter, the trocar with a 5-mm suction nozzle inside connected to another suction machine is introduced into the cannula and, by steady pressure, is pushed into the cyst along with the cannula. Any fluid spillage on puncture of the cyst wall is immediately suctioned either into the body of the hollow trocar through its fenestrated tip and then into the suction cannula or into the outer cannula and thence, into the suction side-channel. Once the PHS enters into the hydatid cyst, the trocar is removed and the cavity is irrigated through the main channel while continuous suction is simultaneously maintained all the time. In this way, fragments of laminated membrane, daughter cysts, and debris are easily removed. Once the retaining fluid is clear, CO2 is insufflated at low pressure (3mm Hg to 4 mm Hg), and the telescope is introduced into the cavity through the cannula to visualize the interior for any overt cyst-biliary communication. In the absence of overt cyst-biliary communication (verified by the absence of bile staining in the suctioned fluid and nonvisualization of the opening within the cyst cavity), 0.5% cetrimide is instilled into the cyst cavity as a scolicidal agent. After 10 minutes, the scolicidal agent is suctioned and the cyst is marsupialized. In case of overt signs of cyst-biliary communication, use of a scolicidal agent is avoided and after marsupialization, the opening is sutured closed with 3– 0 Vicryl. Omentoplasty is done for all cases. The drainage tube is kept near the cyst.
Postoperatively, the cyst size is monitored by US at 2 weeks and 1, 3, 6, 12, 18, and 24 months. If clinically indicated, US is repeated at shorter intervals. CT scan is performed if any complication/recurrence is suspected. The primary end points were defined as complete cyst collapse by US at the end of the procedure, disappearance of cyst cavity or at least 50% reduction in cyst size at follow-up imaging, and disappearance of complications, such as pain, cystobiliary fistulas, vascular or biliary compression, and infection. The secondary end points of the study were recurrence of cyst cavity to >50% of its initial size, vascular or biliary compression, fistulas, pain, and infection within 2 years after surgery, death, withdrawal from the study, or loss to follow-up.
RESULTS
Since 1997, 66 patients have been operated on with PHS. The distribution of the patients regarding age and sex is summarized in Table 1.
Table 1.
Age and Sex of 66 Patients Operated On With the Palanivelu Hydatid System
| Youngest Patient | 14 years |
| Oldest Patient | 64 years |
| Mean Age | 38.6 years |
| Males | 55 (83.3%) |
| Females | 11 (16.7%) |
The majority of patients (51.5%) presented with pain. The mode of presentation of the patients is listed in Table 2.
Table 2.
Symptoms Leading to Discovery of Hydatid Disease
| Abdominal Pain | 34 (51.5%) |
| Nausea, Dyspepsia | 15 (22.7%) |
| Abdominal Swelling/Mass | 13 (19.7%) |
| Accidental Discovery | 3 (4.6%) |
| Fever | 1 (1.5%) |
Most of the patients (N=60, 90.9%) had only a single cyst, while 6 patients (9.1%) had 2 cysts. The right lobe of the liver was more commonly involved (n=36, 54.5%) than was the left lobe (n=26, 39.4%). Cysts were bilateral in 4 cases (6.1%). Fifty-four patients (81.9%) had uncomplicated cysts. Of the remaining, 9 patients (13.6%) presented with cyst-biliary communication, one (1.5%) was a case of secondarily infected cyst and 2 cases (3%) were recurrent cysts. Out of the recurrent cysts, one had recurred after a laparoscopic attempt at removal elsewhere, while the other case was following open hydatid surgery (Table 3).
Table 3.
Clinicopathology
| Single Cyst | 60 (90.9%) |
| Multiple Cysts | 6 (9.1%) |
| Site—Right Lobe of Liver | 36 (54.5%) |
| Site—Left Lobe of Liver | 26 (39.4%) |
| Site—Bilateral | 4 (6.1%) |
| Uncomplicated Cysts | 54 (81.9%) |
| Complicated Cysts | 12 (18.1%) |
| Cyst-Biliary Communication | 9 (13.6%) |
| Secondary Infection | 1 (1.5%) |
| Recurrent Cysts | 2 (3%) |
In 55 patients (83.3%), simply evacuation of the hydatid cyst by PHS was done. In 9 cases (13.7%), this was followed by left lobectomy, as the cysts were large, occupying almost the entire left lobe of the liver. The hepatectomy was performed laparoscopically using a Harmonic scalpel. Transcystic fenestration of underlying cyst was carried out in 2 (3%) of the 6 patients with multiple cysts while the other 4 were dealt with by separate insertions of PHS into individual cysts. The remnant cavity was treated by omentoplasty (Table 4). Omentoplasty was performed by inserting a plug of omentum into the remnant cavity and suturing it intracorporeally with the edge of the cavity with Vicryl 2– 0 interrupted sutures.
Table 4.
Type of Surgery
| Evacuation and Marsupialisation | 55 (83.3%) |
| Evacuation and Lobectomy | 9 (13.7%) |
| Transcystic Fenestration | 2 (3%) |
| Repeated Separate Insertion of Palanivelu Hydatid System | 4 (6%) |
The average duration of the surgery was 52 minutes (range, 36 to 94). None of the patients experienced intraoperative anaphylactic shock.
Postoperatively, 2 patients (3%) had infection, and 9 patients (13.7%) had a minor biliary leak that stopped draining by 5 days to 7 days (Table 5).
Table 5.
Types of Complications
| Grade* | n |
|---|---|
| I | 2 (3%) |
| IIA | 9 (13.7%) |
| IIB | 0 |
| III | 0 |
| IV | 0 |
Classification by Clavien et al.27 Grade I: alterations from the ideal postoperative course, nonlife-threatening and with no lasting disability. Complications of this grade necessitate only bedside procedures and do not significantly extend hospital stay. Grade II: complications that are potentially life-threatening but without residual disability. A subdivision is made according to the requirement for invasive procedures (IIB). Grade III: complications resulting in residual long-term disability, including organ resection or persistence of life-threatening conditions. Grade IV: complications leading to patient death.
Of the 66 patients, regular follow-up has been maintained in 52 patients with an average follow-up period of 5.8 years. There have been no recurrences to date.
DISCUSSION
Physicians worldwide should be aware of the presentation and management of hydatid disease, which is endemic to certain regions, as they may encounter it sporadically due to increased travel and migration.1 Though the common mode of infection is the unhygienic practice of consuming unwashed or improperly washed infected raw fruits and vegetables, direct contact with infected dogs is also another means of contracting the disease, especially in children.28 The youngest patient in the study was 14 years old though the average age of 38.6 years was in keeping with the average age of presentation in endemic areas.29–32 However in nonendemic areas, all the age groups are usually equally affected with the average age of presentation being older.28,33 Males were predominantly affected in the study though other studies have reported equal infestation in both sexes28–30 or predominant female infestation.31,33 Abdominal pain was the most common mode of presentation (51.5% of cases) in this study, which has also been reported by other authors.31,33 Liver hydatidosis is one of the most common causes of acute abdomen in endemic regions. Echinococcal infestation should be suspected in patients who present with an abdominal mass, pain, fever, jaundice, or anaphylaxis.34 However, in nonendemic countries, most of the cases are asymptomatic and are detected fortuitously.1,28,34
The most common pathology was a single cyst in the right lobe of the liver. Similar findings have also been reported by other authors.1,29,33 Ultrasonography and CT are both effective imaging modalities for the detection of liver hydatid disease.28,34 US is particularly useful for the detection of cystic membranes, septa, and hydatid sand, while CT best demonstrates cyst wall calcification and cyst infection.35 Certain features in US, CT, or magnetic resonance imaging (MRI) may warn of biliary communication or impending cyst rupture.30,35 Biliary involvement may be confirmed by MRI or endoscopic retrograde cholangiography (ERC).1,34 Ultrasonographic appearances have also formed the basis of classification of liver hydatid cysts by various authorities like Gharbi,36 WHO,18 and Milicevic.37 Serologic tests have a sensitivity of 65% to 90%28,34 but are not routinely performed in our institution.
Surgery remains the mainstay of treatment for hepatic echinococcosis. Several nonsurgical options have been explored. In endemic countries with scarce surgical resources, a percutaneous approach of aspiration, injection and reaspiration (PAIR) has been advocated by WHO in select cases.18 Modifications of this technique have been developed to address a few of its shortcomings. Saremi38 described a percutaneous approach in which a special cutting instrument is used to fragment and evacuate daughter cysts and laminated membrane while the cavity is continuously irrigated with scolecidals. Percutaneous evacuation of cyst content (PEVAC) using a large bore catheter has been advocated by Schipper et al.6 Drug therapy in the form of oral albendazole is given for specific conditions in liver hydatid, viz.1 widely disseminated hydatid disease,2 localized disease in poor surgical risk patients,3 ruptured cysts, and4 patients in whom significant intraoperative spillage has occurred.34,37 A variety of surgical procedures are done for hydatid cysts of the liver, which are tailored to suit each individual case. These include marsupialization, closed total cystectomy, partial pericystectomy, partial pericystectomy with capitonnage, modified capitonnage, partial pericystectomy with omentoplasty, and typical and atypical liver resections.1,28,34,37,39
The first report of laparoscopic treatment of hydatid cyst of the liver was published in 199440 followed soon thereafter by the first report of anaphylactic shock complicating laparoscopic treatment of hydatid cysts of the liver.41 In fact, an exaggerated fear of anaphylaxis seemed to discourage surgeons from more widely adopting minimal access techniques for the treatment of hydatid cysts.26 However, gradually reports started appearing in the world literature detailing laparoscopic management of liver hydatid disease.19–25,42–45 The indications, contraindications, advantages, and disadvantages of this technique have been elucidated46,47 In our study, we have performed various procedures laparoscopically, viz, evacuation and marsupialization, transcystic fenestration, and left lobectomy. The indications of these procedures are summarized in Table 6. The remnant cyst was dealt with by omentoplasty. We have used the Palanivelu Hydatid System (PHS), a specially designed trocar to obtain a totally contamination-free management of liver hydatid disease. Various laparoscopic techniques described are total pericystectomy, puncture and aspiration of contents followed by marsupialization, unroofing and drainage, unroofing and omentoplasty, and omentoplasty using helical fasteners.22,24,42,43,45,47–49 One of the problems faced in laparoscopic treatment of liver hydatid cysts is the difficulty in evacuating the particulate contents of the cyst, the daughter cysts, and laminated membrane. Various instruments have been described to evacuate the contents of hydatid cysts.21,50–55 Bickel et al21 initially advocated the use of a large transparent beveled cannula. Later on, they modified the technique somewhat by creating a continuous vacuum inside the cannula while its tip was firmly adhered to the cyst wall.50 Saglam51 described a perforator-grinder-aspirator apparatus designed specifically for the evacuation of hydatid cysts. A similar aspirator-grinder apparatus was described by Alper et al.52 Kayaalp53 directly inserted a laparoscopic trocar into the hydatid cyst but reported greater success for anterior and unilocular cysts than for posterior and multi-locular cysts. Al-Shareef et al54 used a liposuction cannula to evacuate hydatid cysts. Zengin et al55 used another perforator and aspirator called the “per-fore-aspirator.” Of all these, the isolated hypobaric technique described by Bickel et al50 is the only one that has attempted to deal with the problem of spillage. PHS not only prevents any spillage of hydatid fluid but also assists complete evacuation of the cyst content and allows intracystic magnified visualization for cyst-biliary communication. We have not had any experience with the devices advocated by other authors.
Table 6.
Indications for Performing Procedures
| Indication | Procedure |
|---|---|
| Single cyst | Evacuation with Palanivelu Hydatid System |
| Two or more cysts separated with normal liver tissue in between | Multiple insertions of Palanivelu Hydatid System |
| Two or more cysts lying immediately adjacent to each other | Transcystic fenestration |
| Large cyst occupying an entire liver segment/lobe | Segmentectomy/hepatectomy/lobectomy |
Postoperatively, 2 of our patients had infections (Clavien type I complication), while 9 of the patients had minor biliary leakage (Clavien type II complication). All the infections and leakages responded to conservative management. Complications seen in open surgery include pleural effusions, infections, biliary fistulae, subdiaphragmatic collection, liver abcesses.5,37 Thus, with laparoscopic management, the severity of complications decreases as compared with that in open surgery.
With an average follow-up of 5.8 years, we have not had any recurrences. Various reports in the literature reveal a recurrence rate varying from 0.9% to 22% for open surgery.56,57 Adequate data are absent from the literature regarding recurrence following the minimally invasive approach.
We have found PHS highly effective and safe for the laparoscopic management of hydatid disease. Due to the freedom from spillage of hydatid fluid, the risk of anaphylactic shock is minimal.
CONCLUSION
We recommend PHS for management of hepatic hydatid disease. We have found its efficacy to be optimum for preventing spillage, evacuating contents of hydatid cysts, performing transcystic fenestration, and for dealing with cyst-biliary communications. The only limitation of this system is related to the anatomical relation of the cyst. At present, we do not recommend this technique for posteriorly located cysts, small cysts, and cysts deep within the hepatic parenchyma.
References:
- 1. Huizinga WKJ, Grant CS, Daar AS. Hydatid disease. In: Morris PJ, Wood WC. eds. Oxford Textbook of Surgery. 2nd ed. New York, NY: Oxford University Press; 2000; 3298–3305 [Google Scholar]
- 2. Schantz PM, Schwabe C. Worldwide status of hydatid disease control. J Am Vet Assoc. 1969; 155: 2104–2121 [PubMed] [Google Scholar]
- 3. King CH. Cestodes (tapeworms). In: Mandell GL, Bennett JE, Dolin R. Principles and Practice of Infectious Diseases. 4th ed. New York, NY: Churchill Livingstone; 1995; 2544–2553 [Google Scholar]
- 4. Gomez R, Marcello M, Moreno E, et al. Incidence and surgical treatment of extra-hepatic abdominal hydatidosis. Rev Esp Enferm Dig. 1992; 82: 100–103 [PubMed] [Google Scholar]
- 5. Amr SS, Amr ZS, Jitawi S, Annab H. Hydatidosis in Jordan: an epidemiological study of 306 cases. Ann Trop Med Parasitol. 1994; 88: 623–627 [DOI] [PubMed] [Google Scholar]
- 6. Kammerer WS, Schantz PM. Echinococcal disease. Infect Dis Clin North Am. 1993; 7: 605–618 [PubMed] [Google Scholar]
- 7. Magistrelli P, Masetti R, Coppola R, et al. Surgical treatment of hydatid disease of the liver: a 20-year experience. Arch Surg. 1991; 126: 518–522 [DOI] [PubMed] [Google Scholar]
- 8. Nahmias J, Goldsmith R, Soibelman M, et al. Three to seven year follow-up after albendazole treatment of 68 patients with cystic echinococcosis (hydatid disease). Ann Trop Med Parasitol. 1994; 88: 295–304 [DOI] [PubMed] [Google Scholar]
- 9. Luchi S, Vincenti A, Messina F, et al. Albendazole treatment of human hydatid tissue. Scand J Infect Dis. 1997; 29: 165–167 [DOI] [PubMed] [Google Scholar]
- 10. Mohamed AE, Yasawy MI, Al Karawi MA. Combined albendazole and praziquantel versus albendazole alone in the treatment of hydatid disease. Hepatogastroenterology. 1998; 45: 1690–1694 [PubMed] [Google Scholar]
- 11. Franchi C, Di vico B, Teggi A. Long-term evaluation of patients with hydatidosis treated with benzimidazole carbamates. Clin Infect Dis. 1999; 29: 304–309 [DOI] [PubMed] [Google Scholar]
- 12. Horton RJ. Albendazole in treatment of human cystic echinococcosis: 12 years experience. Acta Trop. 1997; 64: 79–93 [DOI] [PubMed] [Google Scholar]
- 13. Aeberhard P, Fuhrimann R, Strahm P, Thommen A. Surgical treatment of hydatid disease of the liver: an experience from outside the endemic area. Hepatogastroenterology. 1996; 43: 627–636 [PubMed] [Google Scholar]
- 14. Alfieri S, Doglietto GB, Pacelli F, et al. Radical surgery for liver hydatid disease: study of 89 consecutive patients. Hepatogastroenterology. 1997; 44: 496–500 [PubMed] [Google Scholar]
- 15. Uravic M, Stimac D, Lenac T, et al. Diagnosis and treatment of liver hydatid disease. Hepatogastroenterology. 1998; 45: 2265–2269 [PubMed] [Google Scholar]
- 16. Men S, Hekimoglu B, Yucesoy C, et al. Percutaneous treatment of hepatic hydatid cysts: an alternative to surgery. Am J Roentgenol. 1999; 172: 83–89 [DOI] [PubMed] [Google Scholar]
- 17. Ustunsoz B, Akhan O, Kamiloglu MA, et al. Percutaneous treatment of hydatid cysts of the liver: long-term results. Am J Roentgenol. 1999; 172: 91–96 [DOI] [PubMed] [Google Scholar]
- 18. Brunetti E, Filice C, Macpherson C, et al. PAIR: Puncture, Aspiration, Injection, Re-Aspiration. An option for the treatment of Cystic Echinococcosis. WHO/EMC web site Available at: www.who.int/emc-documents/zoonoses/whocdscsraph20016.html Accessed on October 15, 2004
- 19. Guibert L, Gayral F. Laparoscopic pericystectomy of a liver hydatid cyst. Surg Endosc. 1995; 9: 442–443 [DOI] [PubMed] [Google Scholar]
- 20. Sever M, Skapin S. Laparoscopic pericystectomy of liver hydatid cyst. Surg Endosc. 1995; 9: 1125–1126 [DOI] [PubMed] [Google Scholar]
- 21. Bickel A, Eitan A. The use of a large transparent cannula, with a beveled tip, for safe laparoscopic management of hydatid cysts of liver. Surg Endosc. 1995; 9: 1304–1305 [DOI] [PubMed] [Google Scholar]
- 22. Khoury G, Jabbour-Khoury S, Bikhazi K. Results of laparoscopic treatment of hydatid cysts of the liver. Surg Endosc. 1996; 10: 57–59 [DOI] [PubMed] [Google Scholar]
- 23. Bickel A, Daud G, Urbach D, et al. Laparoscopic approach to hydatid liver cysts. Is it logical? Physical, experimental, and practical aspects. Surg Endosc. 1998; 12: 1073–1077 [DOI] [PubMed] [Google Scholar]
- 24. Ertem M, Uras C, Karahasanoglu T, et al. Laparoscopic approach to hepatic hydatid disease. Dig Surg. 1998; 15: 333–336 [DOI] [PubMed] [Google Scholar]
- 25. Verma GR, Bose SM. Laparoscopic treatment of hepatic hydatid cyst. Surg Laparosc Endosc. 1998; 8: 280–282 [PubMed] [Google Scholar]
- 26. Yaghan R, Heis H, Bani-Hani K, et al. Is fear of anaphylactic shock discouraging surgeons from more widely adopting percutaneous and laparoscopic techniques in the treatment of liver hydatid cyst? Am J Surg. 2004; 187 (4): 533–537 [DOI] [PubMed] [Google Scholar]
- 27. Clavien PA, Sanabria JR, Strasberg SM. Proposed classification of complications of surgery with examples of utility in cholecystectomy. Surgery. 1992; 111: 518–526 [PubMed] [Google Scholar]
- 28. Barnes SA, Lillemoe KD. Liver abscess and hydatid cyst disease. In: Zinner MJ, Schwartz SI, Ellis H. eds. Maingot's Abdominal Operations. 10th ed. Stamford, CT: Appleton & Lange, 1997; 1534–1545 [Google Scholar]
- 29. Cohen H, Paolillo E, Bonifacino R, et al. Human cystic echinococcosis in a Uruguayan community: a sonographic, serologic, and epidemiologic study. Am J Trop Med Hyg. 1998; 59 (4): 620–627 [DOI] [PubMed] [Google Scholar]
- 30. Lewall DB, Nyak P. Hydatid cysts of the liver: two cautionary signs. Br J Radiol. 1998; 71: 37–41 [DOI] [PubMed] [Google Scholar]
- 31. Chautems R, Buhler L, Gold B, et al. Long term results after complete or incomplete surgical resection of liver hydatid disease. Swiss Med Wkly. 2003; 133: 258–262 [DOI] [PubMed] [Google Scholar]
- 32. Schipper HG, Lameris JS, van Delden OM, et al. Percutaneous evacuation (PEVAC) of multivesicular echinococcal cysts with or without cystobiliary fistulas which contain non-drainable material: first results of a modified PAIR method. Gut. 2002; 50: 718–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Niscigorska J, Sluzar T, Marczewska M, et al. Parasitic cysts of the liver—practical approach to diagnosis and differentiation. Med Sci Monit. 2001; 7 (4): 737–741 [PubMed] [Google Scholar]
- 34. Beecherl EE, Bigam DL, Langer B, et al. Cystic diseases of the liver. In: Zuidema GD, Yeo CJ. eds. Shackelford's Surgery of the Alimentary Tract. 5th ed. Vol III, Philadelphia, PA: WB Saunders Company; 2000; 452–460 [Google Scholar]
- 35. Pedrosa I, Saiz A, Arrazola J, et al. Hydatid disease: radiologic and pathologic features and complications. Radiographics. 2000; 20 (3): 795–817 [DOI] [PubMed] [Google Scholar]
- 36. Gharbi HA, Hassine W, Brauner MW, et al. Ultrasound examination of the hydatid liver. Radiology. 139: 459–463 [DOI] [PubMed] [Google Scholar]
- 37. Milicevic MN. Hydatid disease. In: Blumgart LH, Fong Y. eds. Surgery of the Liver and Biliary Tract. 3rd ed. Philadelphia, PA: WB Saunders Company Ltd; 2002; 1167–1204 [Google Scholar]
- 38. Saremi F. Percutaneous drainage of hydatid cysts: use of a new cutting device to avoid leakage. AJR. 1992; 158: 83–85 [DOI] [PubMed] [Google Scholar]
- 39. Filippou DK, Kolimpiris C, Anemodouras N, Rizos S. Modified capitonage in partial cystectomy performed for liver hydatid disease: report of 2 cases. BMC Surg. 1992; 4: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bickel A, Loberant N, Shtamler B. Laparoscopic treatment of hydatid cyst of the liver: initial experience with a small series of patients. J Laparoendosc Surg. 1994; 4 (2): 127–133 [DOI] [PubMed] [Google Scholar]
- 41. Khoury G, Jabbour-Khoury S, Soueidi A, et al. Anaphylactic shock complicating laparoscopic treatment of hydatid cysts of the liver. Surg Endosc. 1998; 12 (5): 452–454 [DOI] [PubMed] [Google Scholar]
- 42. Massoud WZ. Laparoscopic excision of a single hepatic hydatid cyst. Int Surg. 1996; 81 (1): 9–13 [PubMed] [Google Scholar]
- 43. Khoury G, Geagea T, Hajj A, et al. Laparoscopic treatment of hydatid cysts of the liver. Surg Endosc. 1994; 8 (9): 1103–1104 [DOI] [PubMed] [Google Scholar]
- 44. Emelianov SI, Khamidov MA. Laparoscopic treatment of hydatid liver cysts. Khirurgiia (Mosk). 2000; ( 11): 32–34 [PubMed] [Google Scholar]
- 45. Manterola C, Fernandez O, Munoz S. Laparoscopic pericystectomy for liver hydatid cysts. Surg Endosc. 2002; 16 (3): 521–524 [DOI] [PubMed] [Google Scholar]
- 46. Sayek I, Cakmakei M. Laparoscopic management of echinococcal cysts of the liver. Zentralbl Chir. 1999; 124 (12): 1143–1146 [PubMed] [Google Scholar]
- 47. Katkhouda N, Trussler A. Laparoscopic surgery of the liver. In: Zucker KA. ed. Surgical Laparoscopy. 2nd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2001; 211–220 [Google Scholar]
- 48. Seven R, Berber E, Mercan S, et al. Laparoscopic treatment of hepatic hydatid cysts. Surgery. 2000; 128 (1): 36–40 [DOI] [PubMed] [Google Scholar]
- 49. Altinli E, Saribeyoglu K, Pekmezci S, et al. An effective omentoplasty technique in laparoscopic surgery for hydatid disease of the liver. JSLS. 2002; 6 (4): 323–326 [PMC free article] [PubMed] [Google Scholar]
- 50. Bickel A, Loberant N, Singer-Jordan J, et al. The laparoscopic approach to abdominal hydatid cysts: a prospective nonselective study using the isolated hypobaric technique. Arch Surg. 2001; 136 (7): 789–795 [DOI] [PubMed] [Google Scholar]
- 51. Saglam A. Laparoscopic treatment of liver hydatid cysts. Surg Laparosc Endosc. 1996; 6 (1): 16–21 [PubMed] [Google Scholar]
- 52. Alper A, Emre A, Hazar H, et al. Laparoscopic surgery of hepatic hydatid disease: initial results and early follow-up of 16 patients. World J Surg. 1995; 19 (5): 725–728 [DOI] [PubMed] [Google Scholar]
- 53. Kayaalp C. Evacuation of hydatid liver cysts using laparoscopic trocar. World J Surg. 2002; 26 (11): 1324–1327 [DOI] [PubMed] [Google Scholar]
- 54. Al-Shareef Z, Hamour OA, Al-Shlash S, et al. Laparoscopic treatment of hepatic hydatid cysts with a liposuction device. JSLS. 2002; 6 (4): 327–330 [PMC free article] [PubMed] [Google Scholar]
- 55. Zengin K, Unal E, Karabicak I, et al. A new instrument, the “Perfore-Aspirator” for laparoscopic treatment of hydatid cysts of the liver. Surg Laparosc Endosc Percutan Tech. 2003; 13 (2): 80–82 [DOI] [PubMed] [Google Scholar]
- 56. Amir Jahed AK, Fardin R, Farzad A, et al. Clinical echinococcosis. Ann Surg. 1975; 182: 541–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Little JM, Hollands MJ, Ekberg H. Recurrence of hydatid disease. World J Surg. 1988; 12: 700–704 [DOI] [PubMed] [Google Scholar]