Abstract
The sequential changes that occur during the precipitation on mild heating of the unstable hemoglobins, Hb Christchurch, Hb Sydney, Hb Köln, and Hb A, were examined with particular attention to the possibility of an accompanying oxidative process. Hb Christchurch, Hb Sydney, and Hb A precipitated with equal amounts of α- and β-chains and full heme complement. Hb Köln, however, was one-half hemedepleted and showed a slight excess of precipitated β-chains. In all cases the spectrum of the precipitated material was typical of a hemichrome. There was no evidence that sulfhydryl oxidation contributed to the precipitation process. Reduced glutathione was unable to protect the hemoglobin against precipitation, and mixed disulfide formation between the precipitating hemoglobin and glutathione was insignificant, even in the presence of excess glutathione. No blockade of β93 cysteines could be demonstrated in the unstable hemoglobins.
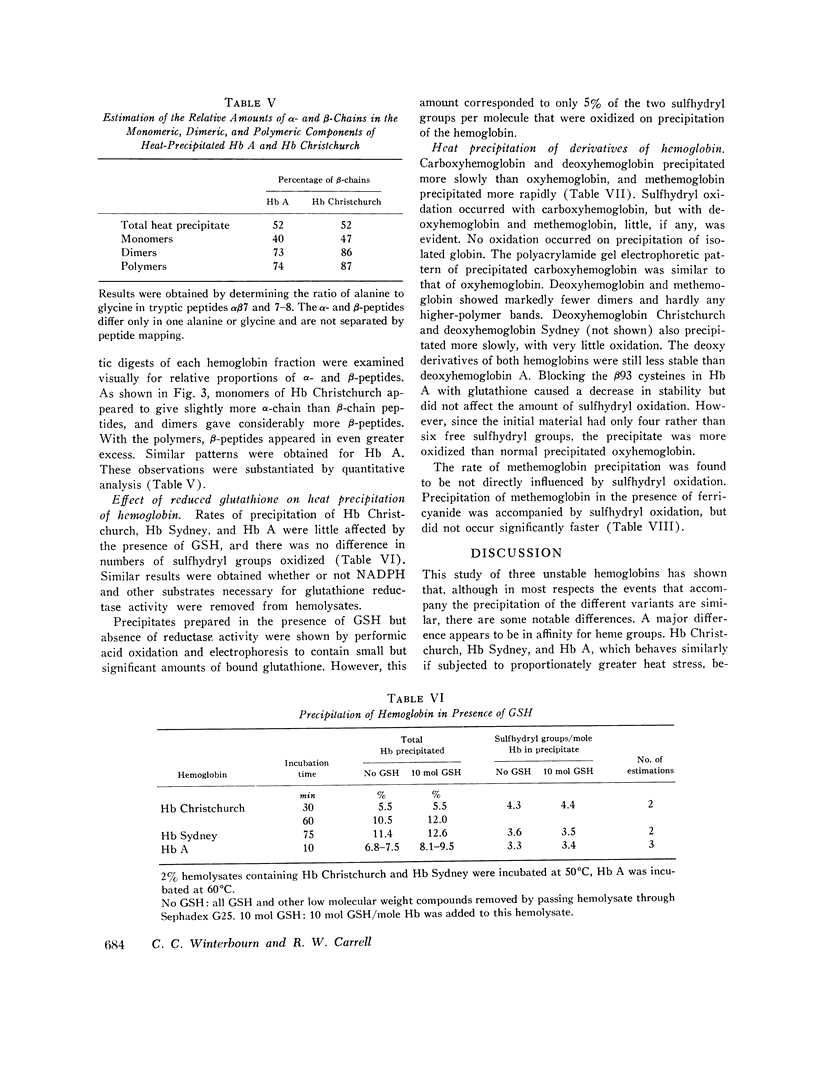
Precipitation of oxyhemoglobin and carboxyhemoglobin in all cases gave nonspecific oxidation of approximately two of the six hemoglobin sulfhydryl groups to give intra- and intermolecular disulfide bonds. Single α- and β-chains, plus polymers of up to five or six chains linked by disulfide bridges, were demonstrated by polyacrylamide gel electrophoresis. This disulfide oxidation was not observed with deoxy- or methemoglobin and did not appear to influence the rate of precipitation. These findings fit the theoretical prediction that autoxidation of oxy- and carboxyhemoglobin is accompanied by formation of a free radical, with the reactions of this free radical being confined intramolecularly.
Together, these results are in keeping with predictions based on the known structural abnormalities of the unstable hemoglobins, all of which result in greater molecular flexibility. Our findings support the conclusion that the usual precipitating event is altered bonding at the heme to give the formation of hemichromes. There is no evidence of an accompanying oxidative process that could pose a threat to the integrity of the red cell.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLEN D. W., JANDL J. H. Oxidative hemolysis and precipitation of hemoglobin. II. Role of thiols in oxidant drug action. J Clin Invest. 1961 Mar;40:454–475. doi: 10.1172/JCI104273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beale D. A partial amino acid sequence for sheep haemoblogin A. Biochem J. 1967 Apr;103(1):129–140. doi: 10.1042/bj1030129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler E. Abnormalities of the hexose monophosphate shunt. Semin Hematol. 1971 Oct;8(4):311–347. [PubMed] [Google Scholar]
- Brown J. R., Hartley B. S. Location of disulphide bridges by diagonal paper electrophoresis. The disulphide bridges of bovine chymotrypsinogen A. Biochem J. 1966 Oct;101(1):214–228. doi: 10.1042/bj1010214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunn H. F. Erythocyte destruction and hemoglobin catabolism. Semin Hematol. 1972 Jan;9(1):3–17. [PubMed] [Google Scholar]
- Carrell R. W., Lehmann H. The unstable haemoglobin haemolytic anaemias. Semin Hematol. 1969 Apr;6(2):116–132. [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- FANELLI A. R., ANTONINI E., CAPUTO A. Studies on the structure of hemoglobin. I. Physicochemical properties of human globin. Biochim Biophys Acta. 1958 Dec;30(3):608–615. doi: 10.1016/0006-3002(58)90108-2. [DOI] [PubMed] [Google Scholar]
- Gabuzda T. G., Laforet M. T., Gardner F. H. Oxidative precipitation of hemoglobin H and its relation to reduced glutathione. J Lab Clin Med. 1967 Oct;70(4):581–594. [PubMed] [Google Scholar]
- Honig G. R., Green D., Shamsuddin M., Vida L. N., Mason R. G., Gnarra D. J., Maurer H. S. Hemoglobin Abraham Lincoln, beta32 (B14) leucine leads to proline. An unstable variant producing severe hemolytic disease. J Clin Invest. 1973 Jul;52(7):1746–1755. doi: 10.1172/JCI107356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huisman T. H., Dozy A. M. Studies on the heterogeneity of hemoglobin. IX. The use of Tris(hydroxymethyl)aminomethanehcl buffers in the anion-exchange chromatography of hemoglobins. J Chromatogr. 1965 Jul;19(1):160–169. doi: 10.1016/s0021-9673(01)99434-8. [DOI] [PubMed] [Google Scholar]
- Jacob H. S., Brain M. C., Dacie J. V. Altered sulfhydryl reactivity of hemoglobins and red blood cell membranes in congenital Heinz body hemolytic anemia. J Clin Invest. 1968 Dec;47(12):2664–2677. doi: 10.1172/JCI105950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob H. S., Brain M. C., Dacie J. V., Carrell R. W., Lehmann H. Abnormal haem binding and globin SH group blockade in unstable haemoglobins. Nature. 1968 Jun 29;218(5148):1214–1217. doi: 10.1038/2181214a0. [DOI] [PubMed] [Google Scholar]
- Jacob H. S. Mechanisms of Heinz body formation and attachment to red cell membrane. Semin Hematol. 1970 Jul;7(3):341–354. [PubMed] [Google Scholar]
- Jacob H. S., Winterhalter K. H. The role of hemoglobin heme loss in Heinz body formation: studies with a partially heme-deficient hemoglobin and with genetically unstable hemoglobins. J Clin Invest. 1970 Nov;49(11):2008–2016. doi: 10.1172/JCI106421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob H., Winterhalter K. Unstable hemoglobins: the role of heme loss in Heinz body formation. Proc Natl Acad Sci U S A. 1970 Mar;65(3):697–701. doi: 10.1073/pnas.65.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra H. P., Fridovich I. The generation of superoxide radical during the autoxidation of hemoglobin. J Biol Chem. 1972 Nov 10;247(21):6960–6962. [PubMed] [Google Scholar]
- Perutz M. F., Lehmann H. Molecular pathology of human haemoglobin. Nature. 1968 Aug 31;219(5157):902–909. doi: 10.1038/219902a0. [DOI] [PubMed] [Google Scholar]
- RIFKIND R. A., DANON D. HEINZ BODY ANEMIA--AN ULTRASTRUCTURAL STUDY. I. HEINZ BODY FORMATION. Blood. 1965 Jun;25:885–896. [PubMed] [Google Scholar]
- Rachmilewitz E. A., Peisach J., Blumberg W. E. Studies on the stability of oxyhemoglobin A and its constituent chains and their derivatives. J Biol Chem. 1971 May 25;246(10):3356–3366. [PubMed] [Google Scholar]
- Rachmilewitz E. A., Peisach J., Bradley T. B., Blumberg W. E. Role of haemichromes in the formation of inclusion bodies in haemoglobin H disease. Nature. 1969 Apr 19;222(5190):248–250. doi: 10.1038/222248a0. [DOI] [PubMed] [Google Scholar]
- Rachmilewitz E. A., White J. M. Haemichrome formation during the in vitro oxidation of Hb Köln. Nat New Biol. 1973 Jan 24;241(108):115–117. doi: 10.1038/newbio241115a0. [DOI] [PubMed] [Google Scholar]
- Rifkind R. A. Heinz body anemia: an ultrastructural study. II. Red cell sequestration and destruction. Blood. 1965 Oct;26(4):433–448. [PubMed] [Google Scholar]
- SHIBATA S., IUCHI I., MIYAJI T., UEDA S., TAKEDA I. HEMOLYTIC DISEASE ASSOCIATED WITH THE PRODUCTION OF ABNORMAL HEMOGLOBIN AND INTRAERYTHROCYTIC HEINZ BODIES. Nihon Ketsueki Gakkai Zasshi. 1963 Apr;26:164–173. [PubMed] [Google Scholar]
- Schneider R. G., Ueda S., Alperin J. B., Brimhall B., Jones R. T. Hemoglobin sabine beta 91 (f 7) leu to pro. An unstable variant causing severe anemia with inclusion bodies. N Engl J Med. 1969 Apr 3;280(14):739–745. doi: 10.1056/NEJM196904032801402. [DOI] [PubMed] [Google Scholar]
- WEISS J. J. NATURE OF THE IRON-OXYGEN BOND IN OXYHAEMOGLOBIN. Nature. 1964 Apr 4;202:83–84. doi: 10.1038/202083b0. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Weed R. I. The importance of erythrocyte deformability. Am J Med. 1970 Aug;49(2):147–150. doi: 10.1016/s0002-9343(70)80069-9. [DOI] [PubMed] [Google Scholar]
- White J. M., Dacie J. V. The unstable hemoglobins--molecular and clinical features. Prog Hematol. 1971;7(0):69–109. [PubMed] [Google Scholar]
- Winterbourn C. C., Carrell R. W. Characterization of Heinz bodies in unstable haemoglobin haemolytic anaemia. Nature. 1972 Nov 17;240(5377):150–152. doi: 10.1038/240150b0. [DOI] [PubMed] [Google Scholar]
- Winterbourn C. C., Carrell R. W. The attachment of Heinz bodies to the red cell membrane. Br J Haematol. 1973 Nov;25(5):585–592. doi: 10.1111/j.1365-2141.1973.tb01770.x. [DOI] [PubMed] [Google Scholar]





