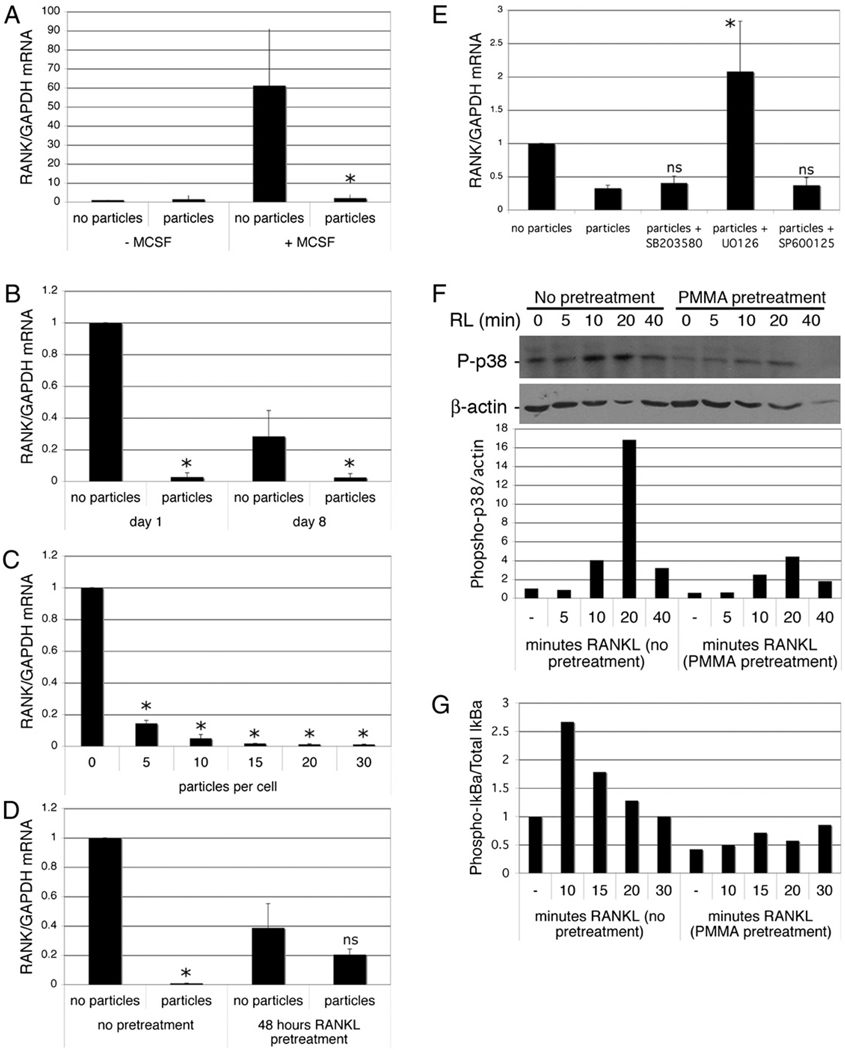
FIGURE 6.
Phagocytosis inhibits expression of RANK. A, Preosteoclasts were isolated and precultured overnight in the absence of M-CSF, then cultured with and without M-CSF (25 ng/ml) and PMMA (20 particles/cell). After 6 h, RNA was extracted and RANK expression measured by qPCR. RANK mRNA levels expressed relative to GAPDH were normalized to the cells treated without M-CSF or particles. n = 3. *p < 0.05 compared with sample with M-CSF and without PMMA. B, Preosteoclasts were cultured for 1 and 8 d with and without PMMA particles (20 particles/cell) in medium supplemented with 25 ng/ml M-CSF. RNA was extracted and RANK expression measured by qPCR. RANK mRNA levels expressed relative to GAPDH were normalized to the day 1 samples without particles. n = 5. *p < 0.01 compared with equivalent sample without particles. C, Dose dependency of particle-mediated repression of RANK expression in preosteoclasts 1 d after particle addition. n = 3. *p < 0.01 compared with equivalent sample without particles. D, Preosteoclasts were cultured for 1 d with and without PMMA particles (20 particles/cell) in medium supplemented with 25 ng/ml M-CSF and 50 ng/ml RANKL with or without a 48-h pretreatment with RANKL. n = 3. *p < 0.01 compared with equivalent sample without particles. E, Preosteoclasts were cultured for 6 h with and without PMMA particles (20 particles/cell) in medium supplemented with 25 ng/ml M-CSF. Where indicated, MAPK inhibitors were added 30 min prior to particles. RNA was extracted and RANK expression measured by qPCR. RANK mRNA levels expressed relative to GAPDH were normalized to the sample without particles. n = 3. *p < 0.05 compared with sample with particles but no inhibitors. F and G, Preosteoclasts were incubated with or without particles for 24 h, after which they were challenged with RANKL. Protein extracts were prepared and analyzed by immunoblotting for phosphorylated p38 and actin (F) or phosphorylated IκBα and total IκBα (G). Quantitation (as described in Materials and Methods) was normalized to control (cells without RANKL or particles). Results from one of two similar experiments are shown.