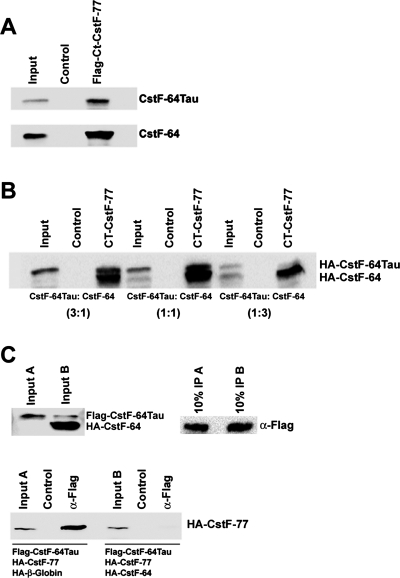
FIGURE 10:
Interaction of CstF-64 and CstF-64Tau with CstF-77. (A) In vitro pull-down. A recombinant C-terminal Flag-tagged fragment of CstF-77 was incubated with in vitro–translated HA-CstF-64 or HA-CstF-64Tau and with α-Flag-agarose followed by subsequent washes and separation by SDS–PAGE. Agarose beads with BSA served as negative control. Coprecipitated proteins were detected by Western blot with α-HA-HRP. (B) In vitro binding competition. Procedures were as described for (A), except that mixtures of in vitro–translated HA-CstF-64 and HA-CstF-64Tau were used as binding substrates. The ratios indicated in brackets refer to the amount of individual template used in the coupled transcription/translation reaction. (C) Inverse in vitro binding competition. In vitro translation products from reactions A (Flag-CstF-64Tau, HA-CstF-77 [full-length], and HA-β-globin) or B (Flag-CstF-64Tau, HA-CstF-77 [full-length], and HA-CstF-64) were incubated with α-Flag-Agarose to precipitate Flag-CstF-64Tau, followed by washes and separation by SDS–PAGE. In the control reactions, the template for Flag-CstF-64Tau was omitted. The presence of Flag-CstF-64Tau and HA-CstF-64 in the reaction was confirmed by Western blot with 10% of the input samples by using an antibody detecting both proteins simultaneously (C-20 antibody, Santa Cruz). The presence of Flag-CstF-64Tau in the precipitate was confirmed by Western blot with 10% of the precipitate by using α-Flag-HRP. Coprecipitated HA-CstF-77 was detected with α-HA-HRP.