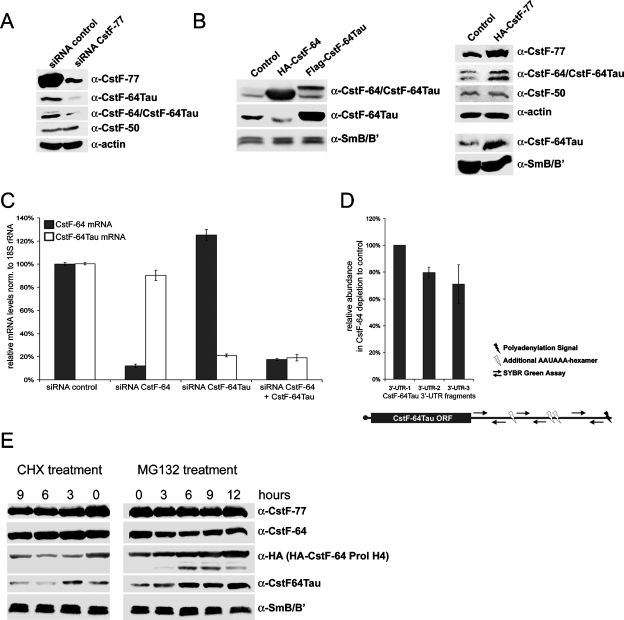
FIGURE 9:
Regulation of CstF-64 and CstF-64Tau. (A) Western blot detecting CstF subunits and β-actin (loading control) upon CstF-77 depletion by RNA interference. Total extracts from cells transfected with a control siRNA or with siRNAs targeting CstF-77 mRNA were separated by SDS–PAGE and CstF-77, CstF-64Tau, CstF-64/CstF-64Tau, and CstF-50 were detected by Western blot. (B) Western blot detecting CstF subunits and SmB/B′ (loading control) in total extracts from cells overexpressing either HA-CstF-64, Flag-CstF-64Tau, or HA-CstF-77. (C) Relative CstF-64 and CstF-64Tau mRNA levels upon depletion of CstF-64, CstF-64Tau, or a combination of both. The relative mRNA levels were measured by qRT-PCR and normalized to 18S rRNA. (D) Alternative polyadenylation qPCR assay for CstF-64Tau upon CstF-64 depletion. Three different SYBR Green assays were designed. The 3′-UTR-1 assay measures all CstF-64Tau mRNA species, whereas 3′-UTR-2 and 3′-UTR-3 are placed after additional AAUAAA hexamers occurring in the CstF-64Tau 3′-UTR. The relative levels are shown as the ratio of CstF-64Tau mRNA levels in control cells to cells depleted of CstF-64 and were normalized to U6 snRNA. (E) Western blot analysis of protein stabilities from cells treated with the translation inhibitor cycloheximide (CHX) or the proteasomal inhibitor MG132 at different time points. Total cell extracts from cells transfected with HA-CstF-64-Prol H4 were separated by SDS–PAGE. Endogenous CstF-77, CstF-64, CstF-64Tau, and SmB/B′ as well as exogenous HA-CstF-64-Prol H4 were detected by Western blotting.