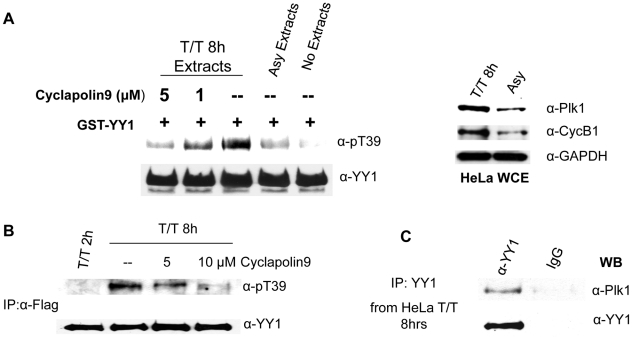
Figure 7. Plk1 phosphorylates YY1 at threonine 39 in vivo.
(A) Western blot analysis of cold in vitro kinase assay reactions using HeLa whole cell extracts (WCE) as the source for kinase activity and bacterially expressed GST-YY1 bound to glutathione beads, as substrate. WCEs were prepared from HeLa cells, asynchronously growing or double-thymidine blocked and released for eight hours (T/T 8 h). Plk1 inhibitor, Cyclapolin 9, was added to the kinase reactions of T/T 8 h extracts at the indicated concentrations. Reactions were separated on SDS-PAGE, transferred to nitrocellulose membrane, and probed with anti-pT39, then anti-YY1 antibody. WCEs were also analyzed on a separate Western blot (right panel), using anti-Plk1 and anti-Cyclin B1 antibodies. Anti-GAPDH was used as a loading control. (B) Flag-YY1 was immunoprecipitated from HeLa-Flag-YY1 cells, synchronized by double-thymidine block and released for eight hours. Cyclapolin 9 (or DMSO, for the negative control for the inhibitor) was added to the cells four hours prior to cell collection. Flag-YY1 was also immunoprecipitated from cell extracts collected two hours after release as a negative control. The resulting Western blot was probed with anti-pT39 and anti-YY1 antibodies. (C) Co-immunoprecipitation of Plk1 with YY1 from WCEs prepared from HeLa cells released for eight hours after double thymidine block. YY1 was immunoprecipitated using an antibody specific for the last 20 amino acids of the YY1 (C-20). IgG was used as a control for the specificity of the immunoprecipitation. The Western blot was probed with anti-Plk1 and then anti-YY1 antibodies.