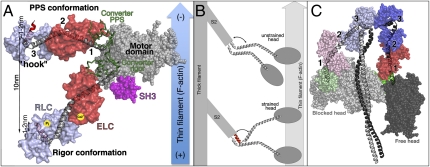
Fig. 1.
(A) Myosin powerstroke is illustrated with the motor domain (including SH3 domain in pink) bound to F-actin and with the lever arm (converter and LCD) in two positions: the primed (prepowerstroke, PPS) and down (Rigor) positions. Three hinges (1–3) are indicated. The newly identified “ankle” hinge involves the characteristic “hook” in the RLC binding region. The angle about the hook can be either obtuse (gray helix) or acute (red helix). (B) The unstrained myosin II dimer binds to F-actin using only one head. Strain applied to the dimer in combination with the structural change in the ankle region may promote binding of the second head to the actin filament. (C) The off state of myosin II promotes asymmetric interactions between the two heads (depicted using similar colors as in A) and the S2 coiled-coil region (gray) of the molecule.