Abstract
Background
This study evaluated the potential of vascularized small intestinal segments for pancreatic islet transplantation.
Methods
We isolated islets from Lewis rats and transplanted these diabetic syngeneic rats. Vascularized segments of small intestine were fabricated with denudation of the mucosal layer followed by implantation of pancreatic islets into the segments. Animal groups were established to determine engraftment, survival and function of islets transplanted into either intestinal segments or portal vein over up to 60 days.
Results
Transplantation of functionally intact pancreatic islets into small intestinal segments was well tolerated. Transplanted islets were rapidly reorganized in intestinal segments with vascularization and expression of insulin and glucagon, throughout the 60-day duration of the studies. Transplantation of islets restored euglycemia in diabetic rats, which was similar to animals receiving islets intraportally. Moreover, animals treated with islet transplants showed normal responses to glucose challenges. Removal of graft-bearing intestinal segments led to recurrence of hyperglycemia indicating transplanted islets were responsible for improved outcomes.
Conclusions
Vascularized intestinal segments supported reorganization, survival and function of transplanted islets with therapeutic efficacy in streptozotocin-treated diabetic rats. The approache described here will be appropriate for studying islet biogenesis, reorganization and function, including for cell therapy applications.
Keywords: Islets, pancreas, portal vein, small intestine, transplantation
Introduction
Type-1 diabetes mellitus is caused by progressive destruction of insulin-producing pancreatic β-cells and affects all age groups. Maintenance of normoglycemia by intense insulin treatment reduces or prevents diabetes complications (1), although this is frequently associated with recurrent episodes of severe hypoglycemia. Moreover, people with brittle or complicated diabetes are often difficult to treat. By contrast, cell therapy by grafting of insulin-producing tissue or cells offers a physiological alternative to insulin alone, as indicated by experiences with islet transplantation into the portal vein (2). On the other hand, transplantation of islets into the portal vein may be associated with complications, e.g., portal thrombosis or subcapsular hematomas (3), and is hampered by significant early losses of transplanted islets.
Development of alternative implantation sites for pancreatic islets should minimize surgical risks and improve therapeutic successes of islet transplantation. Previously, many transplantation sites have been examined for islet transplants, including subcapsular space of the kidney (4), peritoneal cavity for implantation of vascularized biohybrid devices (5), the intramural small bowel site (6–7), gastric submucosa (8), muscle (9), and subcutaneous sites for vascularized devices (10–11).
We considered that transplanted islets will benefit from appropriate microenvironments supporting their engraftment and survival, as well as regulated expression of required genes for maintaining euglycemia. Suitable extracellular matrix components (ECM), cell-cell interactions, and access to vascular supply for endocrine functions should be among the desirable features of such microenvironments. Recently, we demonstrated that an auxiliary liver could be developed in vascularized segments of the small intestine, with synthesis is and secretion of hepatic proteins, i.e., albumin, into the blood. Also, radiotracer studies demonstrated that hepatobiliary excretion pathways were appropriately preserved in transplanted auxiliary liver tissue (12, 13). These studies showed that transplanted liver tissue underwent significant reorganization and angiogenesis, such that isolated small intestinal segments maintained hepatic functions over the long-term (13). Recapitulation of this small intestinal system became of interest to us for pancreatic islets, since tissue revascularization shall provide extensive benefits for islet engraftment, reorganization, survival and functions. Here, we report that pancreatic islets can be successfully transplanted into vascularized small intestinal segments with potential for correcting hyperglycemia in diabetic rats, which offers a new approach for cell therapy in diabetes mellitus.
Materials and Methods
Animals
Inbred male Lewis rats were obtained from Charles River Deutschland (Sulzfeld, Germany). All animal studies were performed at the Department of Clinical Anatomy of Tbilisi State Medical University. Experimental protocols and use of animals was approved by the Institutional Animal Care Committee.
Islet isolation and culture
Islets were isolated from Lewis rats weighing 340 to 400 g as previously described (14). Briefly, the pancreatic duct was occluded near the duodenum and then distended by intraductal injection of 10 mL Hank’s Balanced Salt Solution (HBSS) containing 0.4 mM 4-(2-aminoethyl)-benzene sulfonyl fluoride hydrochloride as trypsin inhibitor, 20 PZ-U of NB1 collagenase and 0.4 DMC-U of neutral protease (Serva Electrophoresis GmbH, Germany). The pancreas was then excised and incubated for 19 to 21 minutes in 37°C shaking water bath. Pancreatic tissue was vortexed on a desktop vortexer at full speed thrice for 10 seconds each. During digestion, tissue samples were evaluated microscopically to monitor dissociation, and undigested material was carefully removed. After three washes with HBSS supplemented with 10% newborn calf serum, the tissue pellet was resuspended in ice-cold University of Wisconsin solution and kept on ice for 30 minutes. Islets were then sedimented in Ficoll (Biochrom, Berlin, Germany). Discontinuous density gradients of Ficoll were formed with HBSS. Dissociated tissue was resuspended in 1.090 g/mL Ficoll and placed at the bottom followed by overlay of 1.077 mg/mL Ficoll and capping with 1.040 g/mL Ficoll. After centrifugation at 800 g for 5 minutes, islets were collected from the interface of 1.077 g/mL and 1.040 g/mL Ficoll. After two washes, islets were picked by hand.
Isolated islets were counted manually. Islet viability was determined by trypan blue dye exclusion. Purity of islets was verified by dithiocarbazone staining.
Assessment of islets function in vitro
Islets were cultured in CMRL 1066 medium containing L-glutamine, 1 mol/L HEPES and 10% fetal calf serum in humidified atmosphere of 95% air, 5% CO2 for 24 hours at 37°C. After overnight culture, insulin secretion was measured by rat insulin immunoassay (ELISA kit; Mercodia, Uppsala, Sweden) and expressed as ng/mL. Glucose-stimulated insulin secretion was determined during static glucose incubation of 20 islets for 120 minutes and this was expressed as stimulation index represented by the ratio of insulin released in the presence of 2.8 mM versus 20 mM glucose.
Surgical fabrication of intestinal segments and islet transplantation
The intestinal segments were created as previously described (12). Briefly, 1-cm long segment of ileum with intact arteriovenous supply was isolated and inverted inside-out by grasping with anatomic forceps. The mucosa of the inverted intestinal segment was scarified by surgical blade until mucosal denudation was complete. The denuded segment was irrigated with normal saline and restored to its normal inside-in position. One end of the separated intestinal segment was then closed with 7-0 Prolene. Fibrin Glue (Tissel; Baxter AG, Vienna, Austria) was applied for hemostasis. Islets were gathered by a Hamilton Syringe (Reno, Nevada, United States), and transferred to a polyethylene catheter, for implantation of 500 islets per recipient (n=6) into the small intestinal segment, followed by application of Fibrin Glue. The other end of the intestinal segment was then closed with 7-0 Prolene. The integrity of the gastrointestinal tract was restored by entero-entero anastomosis with 8-0 surgical silk. The segment containing pancreatic islets was anchored to anterior abdominal wall with Fibrin Glue. After closing the abdominal wall, animals were kept warm until recovery from anesthesia. To verify that islets were functionally intact and viable in vivo, 500 islets were injected into the portal vein in control rats (n=4).
Animals were given amoxicillin 250 mg/L in drinking water for up to 2 weeks after surgical procedures for protection against bacterial infection.
Histopathologic evaluation
Graft-bearing intestinal segments were collected, fixed in 10% neutral buffered formalin and embedded in paraffin. Tissue sections of 5 μm thickness were stained with hematoxylin and eosin (H&E) and Masson’s trichrome for morphological evaluation.
Immunohistochemistry
Expression of insulin and glucagon was analyzed by staining with mouse monoclonal anti-insulin (1:1000) and mouse monoclonal anti-glucagon antibodies (1:2000) (ab6995 and ab10988 respectively, Abcam plc, Cambridge, UK). To demonstrate proliferation in transplanted islet cells, tissue sections were stained with rabbit monoclonal Ki-67 antibody (1:150) (ab16667, Abcam plc, Cambridge, UK). Tissue revascularization was verified by immunostaining with α-smooth muscle actin (SMA) antibody (1:100) (ab18147, Abcam plc, Cambridge, UK). Secondary detection was performed with rabbit-specific goat IgG for Ki-67 (ab6721, Abcam plc, Cambridge, UK) and mouse-specific rabbit IgG for insulin, glucagon and α-SMA (ab6728, Abcam plc, Cambridge, UK) with diaminobenzidine (DAB) substrate (Abcam plc, Cambridge, UK).
Studies of Islet Function
Diabetes was induced in inbred male Lewis rats of 6–9 weeks of age, weighing 160 to 220 g, utilizing one intraperitoneal dose of 65 mg/kg streptozotocin (STZ; Sigma, Steinhein, Germany). Nonfasting blood glucose levels were measured daily during the first week post transplantation, twice per week for the first month, and once per week until the end of the study. Animals were considered hyperglycemic with blood glucose levels ≥350 mg/dL for 2 consecutive days.
Islets were transplanted in diabetic animals 5 to 7 days after STZ treatment. Graft function was defined as non-fasting glycemic levels ≤200 mg/dl. Graft failure was defined as blood glucose levels ≥350 mg/dL for at least 2 consecutive days. To exclude residual function of the native pancreas, graft-bearing intestinal segments were removed 7, 12, 30 and 60 days after initial islet transplantation surgery followed by further monitoring of blood sugar levels. Glucose tolerance test was performed 58 days after islet transplantation to assess the metabolic activity of transplanted islets in intestinal segments. Fasting animals were given 2 g/kg glucose in normal saline intravenously followed by blood glucose measurements after 15, 30, 60 and 120 minutes.
Statistical Analysis
Data were expressed as means ± SE as appropriate. The differences were considered significant when P value was <0.05 with Mann-Whitney test.
RESULTS
Islet Quality Assessment
The number of islets isolated was 1600±76 per donor pancreas. Isolated islets appeared to be morphologically intact, excluded trypan blue dye, and stained with dithiocarbazone, including immediately after isolation, as well as after culture (not shown). The viability of freshly isolated islets was 94%±2%. The glucose stimulation index was 3.1± 0.5.
Engraftment of Islets in Small Intestinal Segment
The surgical procedure to create a vascularized intestinal segment required approximately 40 minutes. Figure 1 demonstrates key components of the surgical procedure. Rats recovered promptly and all animals survived after pancreatic islet transplantation into the intestinal segment. At later times (see below), the reanastomosed small intestine was appeared normal without any evidence of gastrointestinal obstruction and intestinal segments containing transplanted islets showed normal appearance.
Fig. 1. Surgical procedure.
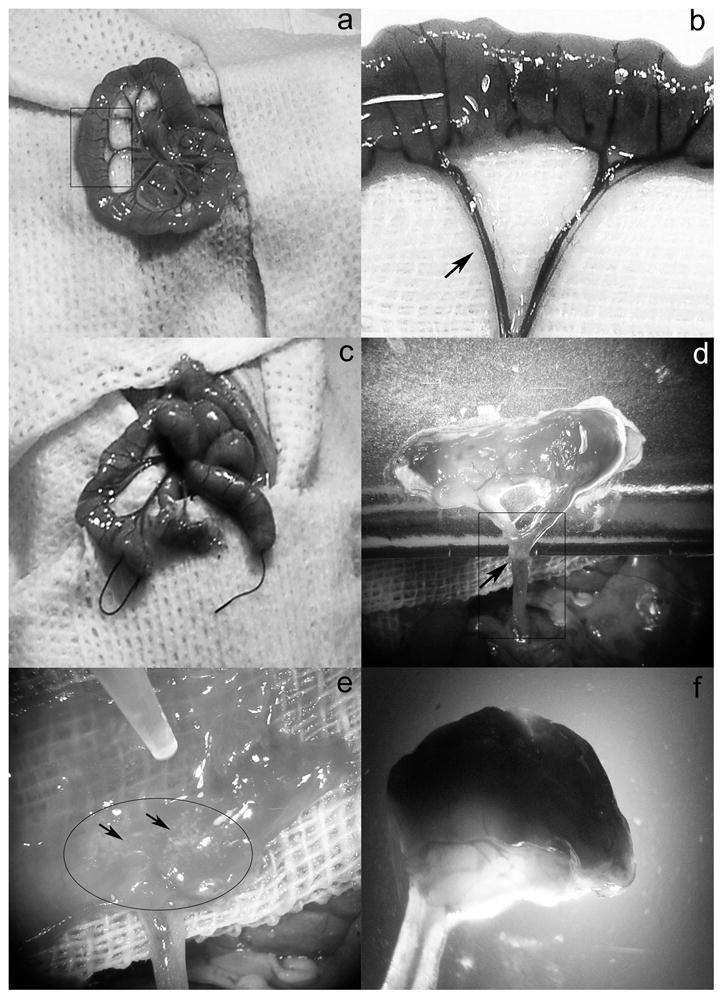
(a, b) Identification of an appropriate small intestinal loop (box in a) with intact arteriovenous pedicle (arrow in b). (c) Separation of the small intestinal segment and (d) closure of one end by a ligature and the final appearance of the segment with intact vascular pedicle (arrow). (e) Showing placement of pancreatic islets on the submucosa of the intestinal segment (arrows). (f) Completion of the small intestinal segment filled with pancreatic islets.
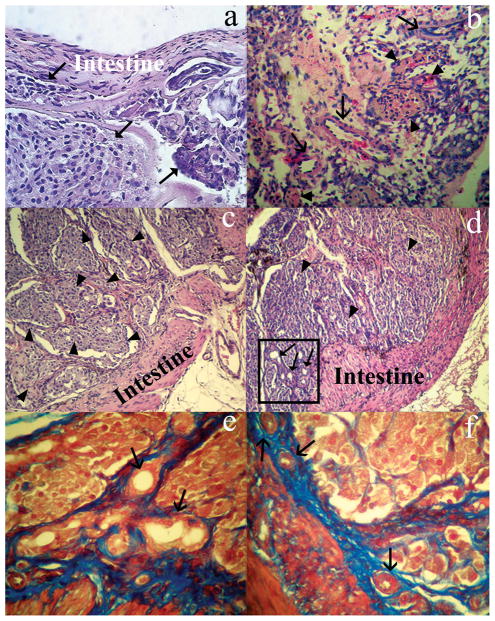
Histological examination of intestinal segments showed that the intestinal mucosa was completely denuded, while the submucosa was well preserved in intestinal segments containing transplanted islets (Fig. 2a). This preservation of intestinal submucosa with capillaries and other structures should have been important for maintaining the viability of the intestinal segments, along with transplanted islets. We found abundant vascular structures throughout the intestinal segments at various periods after islet transplantation. Remarkably, we did not observe inflammatory infiltrates in intestinal segments containing transplanted islets (Fig. 2a–f). The overall morphological structure of transplanted islets, which were embedded within connective tissue in the intestinal segments, appeared to be well preserved over up to 60 days (Figs. 2c–f).
Fig. 2. Histopathological assessment of explanted islet-containing intestinal segments.
(a) Shows the overall organization of islets (arrowheads) bounded by the intestinal wall (intestine) 7 days after their transplantation. Note that the intestinal mucosa is completely denuded and is without obvious inflammatory infiltrates. (b) Shows numerous vascular structures (arrows) throughout the section 12 days after transplantation of islets in the intestinal segment. (c, d) Islets (arrowheads) 60 days after transplantation into the intestinal segment. Abundant vascular structures are indicated in the box. (e, f) Collagen formation stained by blue Masson′s trichrome surrounding islet structures. (a–d) Hematoxilin and Eosin staining; e and f, Masson’s trichrome. Original magnification; a and b, ×200, c and d ×100; and e and f, ×400.
To demonstrate that transplanted islets were functionally capable, we analyzed expression of insulin and glucagon in situ. Insulin and glucagon were expressed in transplanted islets throughout the observation period of 60 days (Fig. 3a–d), at intensity levels similar to donor pancreas (data not shown). Similarly, immunostaining for α-SMA verified presence of mature blood vessels in areas around islets, as well as within the intestinal submucosa (Fig. 3e–3f), which indicated vascularization of transplanted islets. We did not observe any evidence of apoptosis in transplanted islets (not shown). Moreover, we did not detect proliferation in transplanted islets, as indicated by Ki-67 immunostaining in explanted tissues 60 days after islet transplantation (not shown).
Fig. 3. Endocrine and other functions in transplanted islets.
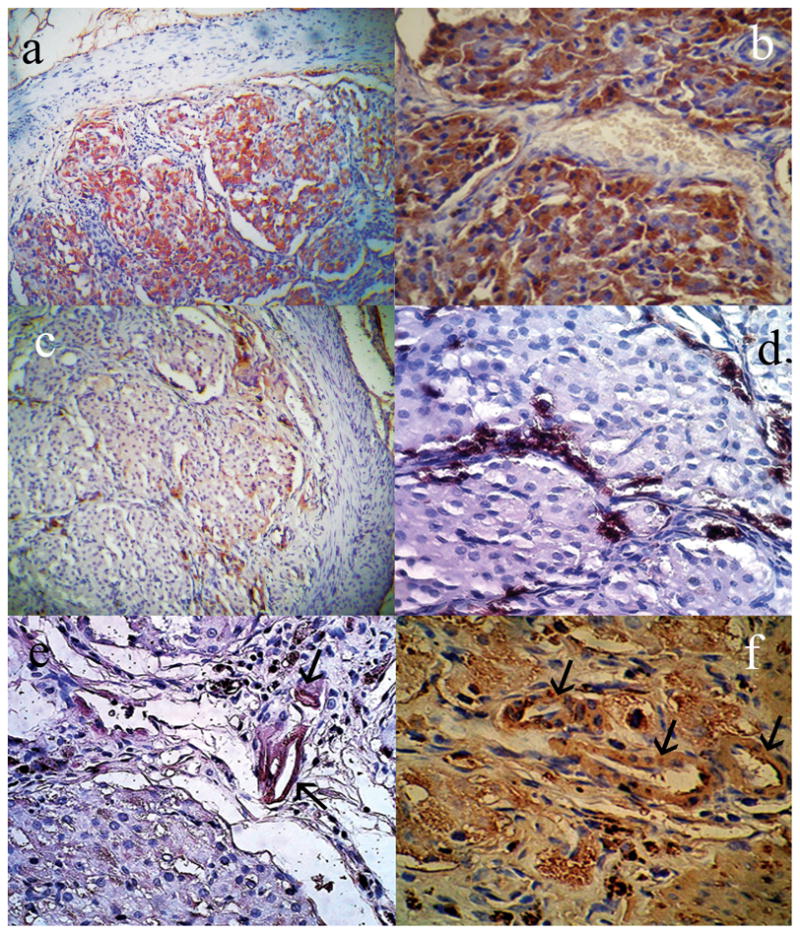
Immunostaining for (a, b) insulin and (c, d) glucagon, as indicated by DAB staining in islet cells transplanted into intestinal segments. α-SMA immunostaining to identify mature blood vessels (arrows) in (e) islets transplanted into intestinal segments, as well as (f) intestinal submucosa. Original magnification a, c, ×200; b, d, e and f ×400.
Posttransplant function
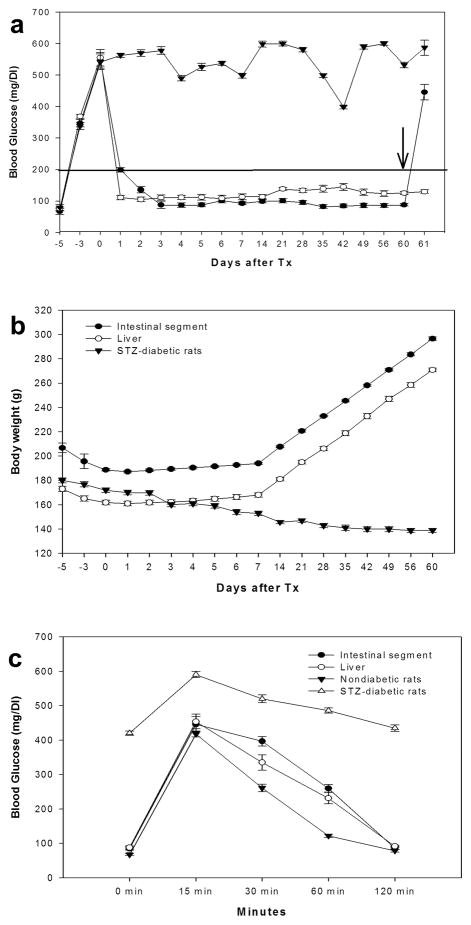
In all diabetic animals subjected to islet transplantation, irrespective of whether islets were transplanted into intestinal segments or portal vein, blood glucose levels returned to normoglycemic ranges within 20 days (Fig. 4a). Consequently, animals showed improvements in clinical condition with steady gains in body weight after islet transplantation (Fig. 4b). On the other hand, when intestinal segments bearing transplanted islets were removed, hyperglycemia invariably returned in all rats within 24 hours after graft removal, confirming that normoglycemia was restored by transplanted islets in the intestinal segments. Glucose tolerance tests revealed excellent functional capacity of transplanted islets. In diabetic rats treated with islets transplanted into the intestinal segment, glucose challenge on day 58 showed rapid return to normoglycemia (0 minutes: 85±12 mg/dL; 15 minutes: 446±54 mg/dL; 30 minutes: 397±35 mg/dL; 60 minutes: 260±28 mg/dL; and 120 minutes: 87±11 mg/dL). This was comparable to normal rats (0 minutes: 68±6 mg/dL; 15 minutes: 418±22 mg/dL; 30 minutes: 261±28 mg/dL; 60 minutes: 122±11 mg/dL; 120 minutes: 79±11 mg/dL), However, diabetic rats without islet transplantation showed abnormal glucose tolerance tests (0 minutes: 420±15 mg/dL; 15 minutes: 590±24 mg/dL; 30 minutes: 520±25 mg/dL; 60 minutes: 486±13 mg/dL; 120 minutes: 435±26 mg/dL) (Fig. 4c).
Fig. 4. Regulation of blood glucose levels and body weight in diabetic rats after islet transplantation.
(a) Shown are animals with small intestinal islet transplants (Group 1, -●-) and intraportal islet transplants (Group 2, -○-) to indicate return of blood glucose levels to <200 mg/dl in both groups. Control STZ-treated diabetic rats without islet transplantation (-▼-) are shown for comparison. Note that hyperglycemia promptly recurred when islet-bearing intestinal segments were removed in animals (arrow), p<0.05, which confirmed that glycemic control was due to transplanted islets. (b) Showing gains in body weight in treated animals after improvement in hyperglycemia. (c) Shows normalization of glucose tolerance tests in diabetic rats after transplantation of islets into either intestinal segment (-●-) or portal vein (-○-). Nondiabetic healthy rats (-▼-) and control STZ-treated diabetic rats (-△-) are shown for comparison.
Discussion
We demonstrated that pancreatic islets could be successfully transplanted into vascularized segments of the small intestine and this restored normoglycemia in STZ-treated diabetic rats. Tissue studies revealed that islets transplanted in intestinal segements had normal morphology, and maintained expression of insulin as well as glucagon, which are both necessary for maintenance of euglycemia. Moreover, normoglycemia was restored in diabetic rats treated with islets transplanted in intestinal segments. Therefore, this tissue-engineering approach will be appropriate for addressing mechanisms in islet biology and for cell therapy in type-1 diabetes.
The impetus for developing novel approaches for islet transplants is driven by limitations with intraportal infusion of islets, which is the commonest procedure at present for islet transplantation. For instance, destruction of 50–80% of islets immediately after intraportal transplantation is a major problem (15). Such early islet losses arise from multiple mechanisms, including onset of inflammation, blood clotting, shear forces generated by portal blood flow, as well as lack of suitable ECM to support islet engraftment and reorganization. Consequently, an excess of islets must be transplanted to achieve insulin independence. On the other hand, transplantation of increased number of islets may result in deleterious biological effects, e.g., release of tissue factor may activate platelets, granulocytes and monocytes with thrombotic events, tissue injury, inflammation, and loss of transplanted islets (16). Among various deleterious mechanisms, the hyperglycemic liver environment in diabetes and exposure to immunosuppressive drugs or drug metabolites in the liver (17) may cause toxicity to β cells, contributing in islet graft loss, as was observed in clinical trials (18–19). In contrast, alternative sites for islet implantation are of interest, as indicated by results of intramuscular islet autotransplantation (20). By contrast, transplantation of islets beneath the kidney capsule in people has not produced convincing beneficial results. It should be noteworthy that the space under the kidney capsule, while adequate for experimental studies in rodents, really is insufficient for people, and limited vascularization in this site leads to generally inadequate vascularization and oxygenation of islets, especially in the deeper parts of the graft (21–22). Certainly, subcutaneous implantation of islets is technically simple, although this produces limited graft survival (23). Similarly, survival of transplanted islets was limited in the spleen, despite its vascularized nature (4). Although studies in animal models examined the utility of islet transplantation under the gastric submucosa and subserosa (8, 24), more work is needed to understand the potential of these locations.
Our interest in vascularized small intestinal segments was generated by our recent experience of transplanted liver tissue with remodeling and revascularization in this setting (13). These studies showed that several growth factors required for angiogenesis and vasculogenesis were expressed in the intestinal wall of the vascularized segment, including vascular endothelial growth factor, fibroblast growth factor-2, hepatocyte growth factor, and transforming growth factor-β, along with several angiogenesis-related genes. This permitted us to consider that mucosal denudation of the intestinal segment will provide opportunities for transplanted islets to receive oxygen from submucosal vessels followed by revascularization of engrafted islets. The findings of rapid islet reorganization, including development of vessels and extracellular matrix system in the grafted tissue were in agreement with islet survival and function in the long-term. Beneficial tissue anchorage to ECM in a microenvironment enriched by trophic growth factors, nutrients and antiapoptotic signals was reproduced by vascularized small intestinal segments, as indicated by the absence of apoptosis in transplanted islets. In the past, numerous studies described the benefits of ECM on islet survival and islet functional capacity (25). Recently, pancreatic islets were found to attach well to the small intestinal submucosa (SIS), leading to improved survival over prolonged periods (26), which was in agreement with our findings, since SIS is naturally enriched in collagen types I, III and VI, glycosaminoglycans (hyaluronic acid, chondroitin sulfate A and B, heparin, heparan sulfate), proteoglycans, fibronectin, etc. Therefore, we considered that the small intestinal segment fabricated from autologous tissues in vivo provided opportunities for graft vascularization, survival and function of transplanted islets.
In conclusion, we demonstrate that pancreatic islets can be successfully transplanted into small intestinal segments. This should encourage further development of this model to enhance islet neogenesis, survival, and function. The tissue-engineering approach described here could eventually be beneficial for cell therapy in diabetes mellitus.
Acknowledgments
This work was supported by a “New Horizons Collaborative Research Initiative Grant” from European Foundation for the Study of Diabetes (EFSD).
SG was supported in part by NIH grants 2P01-DK052956, 2DKR01-46592, 1DKR01-071111, 2P30-DK41296 and U54-RR023467.
Footnotes
Z. K. and E. B. participated in research design; Z. K., S. G., D. B. and E. B. participated in the writing of the manuscript; Z. K. and E. B. participated in the performance of the research; and Z. K., S. G., D. B. and E. B. participated in data analysis.
No conflict of interest.
References
- 1.Graveling AJ, Frier BM. Hypoglycaemia: an overview. Prim Care Diabetes. 2009;3:131–9. doi: 10.1016/j.pcd.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 2.Ricordi C, Strom TB. Clinical islet transplantation: advances and immunological challenges. Nat Rev Immunol. 2004;4:259–000. doi: 10.1038/nri1332. [DOI] [PubMed] [Google Scholar]
- 3.Ricordi C, Inverardi L, Kenyon NS, Goss J, Bertuzzi F, Alejandro R. Requirements for success in clinical islet transplantation. Transplantation. 2005;79:1298–300. doi: 10.1097/01.tp.0000157275.64874.84. [DOI] [PubMed] [Google Scholar]
- 4.Scharp DW, Marchetti P, Swanson C, Newton M, McCullough CS, Olack B. The effect of transplantation site and islet mass on long-term survival and metabolic and hormonal function of canine purified islet autografts. Cell Transplant. 1992;1:245–54. doi: 10.1177/0963689792001002-306. [DOI] [PubMed] [Google Scholar]
- 5.Lanza RP, Borland KM, Lodge P, Carretta M, Sullivan SJ, Muller TE, Solomon BA, Maki T, Monaco AP, Chick WL. Treatment of severely diabetic pancreatectomized dogs using a diffusion-based hybrid pancreas. Diabetes. 1992;41:886–9. doi: 10.2337/diab.41.7.886. [DOI] [PubMed] [Google Scholar]
- 6.Wang XG, Tafra L, Berezniak R, Lloyd RV, Muraika L, Dafoe DC. Effects of cotransplanted fetal liver on fetal pancreas isografts. Transplantation. 1992;53:272–6. doi: 10.1097/00007890-199202010-00004. [DOI] [PubMed] [Google Scholar]
- 7.Tchervenivanov N, Yuan S, Lipsett M, Agapitos D, Rosenberg L. Morphological and functional studies on submucosal islet transplants in normal and diabetic hamsters. Cell Transplant. 2002;11:529–37. [PubMed] [Google Scholar]
- 8.Caiazzo R, Gmyr V, Hubert T, Delalleau N, Lamberts R, Moerman E, Kerr-Conte J, Pattou F. Evaluation of alternative sites for islet transplantation in the minipig: interest and limits of the gastric submucosa. Transplant Proc. 2007;39:2620–3. doi: 10.1016/j.transproceed.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 9.Weber CJ, Hardy MA, Pi-Sunyer F, Zimmerman E, Reemtsma K. Tissue culture preservation and intramuscular transplantation of pancreatic islets. Surgery. 1978;84:166–74. [PubMed] [Google Scholar]
- 10.Pileggi Antonello, Molano R Damaris, Ricordi Camillo, Pileggi A, Molano RD, Ricordi C, Zahr E, Collins J, Valdes R, Inverardi L, et al. Reversal of diabetes by pancreatic islet transplantation into a subcutaneous, neovascularized device. Transplantation. 2006;81:1318–24. doi: 10.1097/01.tp.0000203858.41105.88. [DOI] [PubMed] [Google Scholar]
- 11.Lacy PE, Hegre OD, Gerasimidi-Vazeou A, Gentile FT, Dionne KE. Maintenance of normoglycemia in diabetic mice by subcutaneous xenografts of encapsulated islets. Science. 1991;254:1782–4. doi: 10.1126/science.1763328. [DOI] [PubMed] [Google Scholar]
- 12.Berishvili E, Liponava E, Kochlavashvili N, Kalandarishvili K, Benashvili L, Gupta S, Kakabadze Z. Heterotopic auxiliary liver in an isolated and vascularized segment of the small intestine in rats. Transplantation. 2003;75:1827–32. doi: 10.1097/01.TP.0000065297.56712.C1. [DOI] [PubMed] [Google Scholar]
- 13.Joseph Brigid, Berishvili Ekaterine, Benten Daniel, Kumaran Vinay, Liponava Ekaterine, Bhargava Kuldeep, Palestro Christopher, Kakabadze Zurab, Gupta Sanjeev. Isolated small intestinal segments support auxiliary livers with maintenance of hepatic functions. Nat Med. 2004;10:749–53. doi: 10.1038/nm1057. [DOI] [PubMed] [Google Scholar]
- 14.Brandhorst H, Raemsch-Guenther N, Raemsch C, Friedrich O, Huettler S, Kurfuerst M, Korsgren O, Brandhorst D. The ratio between collagenase class I and class II influences the efficient islet release from the rat pancreas. Transplantation. 2008;85:456–461. doi: 10.1097/TP.0b013e31816050c8. [DOI] [PubMed] [Google Scholar]
- 15.Harlan DM, Kenyon NS, Korsgren O, Roep BO. Immunology of Diabetes Society. Current advances and travails in islet transplantation. Diabetes. 2009;58:2175–84. doi: 10.2337/db09-0476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ozmen L, Ekdahl KN, Elgue G, Larsson R, Korsgren O, Nilsson B. Inhibition of thrombin abrogates the instant blood-mediated inflammatory reaction triggered by isolated human islets: possible application of the thrombin inhibitor melagatran in clinical islet transplantation. Diabetes. 2002;51:1779–84. doi: 10.2337/diabetes.51.6.1779. [DOI] [PubMed] [Google Scholar]
- 17.Desai NM, Goss JA, Deng S, Wolf BA, Markmann E, Palanjian M, Shock AP, Feliciano S, Brunicardi FC, Barker CF, Naji A, Markmann JF. Elevated portal vein drug levels of sirolimus and tacrolimus in islet transplant recipients: local immunosuppression or islet toxicity? Transplantation. 2003;76:1623–5. doi: 10.1097/01.TP.0000081043.23751.81. [DOI] [PubMed] [Google Scholar]
- 18.Ryan EA, Paty BW, Senior PA, Bigam D, Alfadhli E, Kneteman NM, Lakey JR, Shapiro AM. Five-year follow-up after clinical islet transplantation. Diabetes. 2005;54:2060–9. doi: 10.2337/diabetes.54.7.2060. [DOI] [PubMed] [Google Scholar]
- 19.Froud T, Ricordi C, Baidal DA, Hafiz MM, Ponte G, Cure P, Pileggi A, Poggioli R, Ichii H, Khan A, Ferreira JV, Pugliese A, Esquenazi VV, Kenyon NS, Alejandro R. Islet transplantation in type 1 diabetes mellitus using cultured islets and steroid-free immunosuppression: miami experience. Am J Transplant. 2005;5:2037–46. doi: 10.1111/j.1600-6143.2005.00957.x. [DOI] [PubMed] [Google Scholar]
- 20.Rafael E, Tibell A, Rydén M, Lundgren T, Sävendahl L, Borgström B, Arnelo U, Isaksson B, Nilsson B, Korsgren O, Permert J. Intramuscular autotransplantation of pancreatic islets in a 7-year-old child: a 2-year follow-up. Am J Transplant. 2008;8:458–62. doi: 10.1111/j.1600-6143.2007.02060.x. [DOI] [PubMed] [Google Scholar]
- 21.Mattsson G, Jansson L, Carlsson PO. Decreased vascular density in mouse pancreatic islets after transplantation. Diabetes. 2002;51:1362–6. doi: 10.2337/diabetes.51.5.1362. [DOI] [PubMed] [Google Scholar]
- 22.Källskog O, Kampf C, Andersson A, Carlsson PO, Hansell P, Johansson M, Jansson L. Lymphatic vessels in pancreatic islets implanted under the renal capsule of rats. Am J Transplant. 2006;6:680–6. doi: 10.1111/j.1600-6143.2006.01234.x. [DOI] [PubMed] [Google Scholar]
- 23.Trucco M. Regeneration of the pancreatic beta cell. J Clin Invest. 2005;115:5–12. doi: 10.1172/JCI23935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Echeverri GJ, McGrath K, Bottino R, Hara H, Dons EM, van der Windt DJ, Ekser B, Casu A, Houser S, Ezzelarab M, Wagner R, Trucco M, Lakkis FG, Cooper DK. Endoscopic Gastric Submucosal Transplantation of Islets (ENDO-STI): Technique and Initial Results in Diabetic Pigs. Am J Transplant. 2009;9:2485–96. doi: 10.1111/j.1600-6143.2009.02815.x. [DOI] [PubMed] [Google Scholar]
- 25.Ris F, Hammar E, Bosco D, Pilloud C, Maedler K, Donath MY, Oberholzer J, Zeender E, Morel P, Rouiller D, Halban PA. Impact of integrin-matrix matching and inhibition of apoptosis on the survival of purified human beta-cells in vitro. Diabetologia. 2002;45(6):841–50. doi: 10.1007/s00125-002-0840-7. [DOI] [PubMed] [Google Scholar]
- 26.Xiaohui T, Wujun X, Xiaoming D, Xinlu P, Yan T, Puxun T, Xinshun F. Small intestinal submucosa improves islet survival and function in vitro culture. Transplant Proc. 2006;38:1552–8. doi: 10.1016/j.transproceed.2006.02.134. [DOI] [PubMed] [Google Scholar]