Abstract
Background
Although many adolescents experiment with cigarettes, not all who smoke go on to use regularly or develop dependence. This heterogeneity in subsequent cigarette use suggests that there are meaningful individual differences in how cigarettes are initially experienced.
Method
Subjective experiences were retrospectively collected using the Early Smoking Experience (ESE) questionnaire from 2482 young adults participating in the National Longitudinal Study of Adolescent Health. Responses from this genetically informative sample of same-and opposite sex twin-and siblings were examined to determine the structure and magnitude of genetic and environmental influences.
Results
Positive experiences evidenced moderate heritable contributions (35%, 95% CI: 0.19 to 0.49), as did an overall hedonic measure (31%, 95% CI: 0.20 to 0.42) and dizziness (31%, 95% CI: 0.14 to 0.48). Negative experiences evidenced small heritable contributions (18%, 95% CI: 0.04 to 0.78). Individual specific environmental influences were strong and accounted for the remaining proportion of observed variation in these experiences. Multivariate genetic modeling identified a moderately heritable underlying factor (37%, 95% CI: 0.22 to 0.52) that influenced the covariation of diverse subjective experiences and loaded most heavily on dizziness. Positive experiences also evidence residual genetic influences that were uncorrelated with other subjective experiences.
Conclusions
How a person experiences their initial few cigarettes is due to both heritable contributions and environmental experiences unique to the person. The covariation of diverse subjective experiences appears to be due to a heritable latent sensitivity to the chemicals contained in an average cigarette and is best indexed by dizziness.
Introduction
The acquisition of cigarette use during adolescence is likely due to a multitude of factors and is understandably a serious public health problem. Although prevention efforts have impacted cigarette use negatively, many continue to experiment [1]. Through this process, adolescents are exposed to the autonomic and sensory effects of the 4000+ chemicals contained in an average cigarette [2 -5]. These discriminative or ‘subjective’ effects are thought to act as positive and/or negative reinforcements that are associated with later smoking behaviors [5-7]. As cigarette use may promote brain adaptations that are associated with reinforcement and use, investigating the etiology of initial subjective experiences may aid our understanding of initial drug response, the maintenance of cigarette use, as well as inform tobacco prevention programs.
Subjective experiences of initial tobacco exposure often includes dizziness, light-headedness, buzz or a rush, nausea, increased heart pounding, euphoria, relaxation, and sedation. Broadly these are though to reflect the physiological and pharmacological actions of nicotine or the chemical by-products of smoking cigarettes, with differences between individuals ingesting similar quantities reflected in their subjective reports. Across different measures these and other initial experiences reliably cluster into two broad categories that include pleasant and unpleasant [8-11]. Whereas these two factors are weakly or inversely correlated [9, 11, 12], both types of experiences are reported in studies of smokers with little to no previous exposure. Pleasant experiences, which include effects such as head-rush, euphoria, relaxation, increased sociability and energy, are implicated as a risk factor for greater cigarette use and early onset nicotine dependence [9, 12-17]. Conversely, unpleasant or adverse sensations have been associated with inconsistent later use or complete desistence [18]; though such effects are not necessarily prohibitive of progressing to dependence [9, 19].
Despite their limitations, questionnaire-based studies of subjective experiences offer a number of advantages. These include their ease of administration in epidemiological studies, brevity, ability to avoid the ethical issues associated with experimental studies of drug naïve adolescents, and usefulness in assessing environmental influences on initial smoking experiences. Among those available, the most commonly used measure is the Early Smoking Experiences scale (ESE) [9]. The ESE is a nine-item, self-report questionnaire that asks participants to retrospectively rate their initial smoking experiences on a Likert-scale from none (1) to intense (4). Previous research using this scale has supported a two factor model [9, 12, 20], with both pleasant and unpleasant factors demonstrating acceptable internal reliability. Dizziness has been implicated as a particularly informative experience related to subsequent smoking behaviors, shown to load on both pleasant and unpleasant factors, and to demonstrate strong reliability across time [9, 12, 15, 17, 18, 20-22].
Though both experimental- and questionnaire-based studies have documented variation in how initial nicotine exposure is experienced, the sources of that variation remain to be established. Towards that end, twin studies offer a practical methodology for evaluating the importance of environmental and heritable variation on susceptibility. Despite the large number of genetically informative studies of many cigarette use behaviors, there has only been a single study of subjective experiences in a small sample of discordant twins [23]. As the discordant twin design examines whether environmental influences contribute to observed differences between monozygotic twins, the magnitude and nature of genetic and environmental influences on subjective experiences remains unclear. Moreover, given differences in the relationship with later use, it is unclear positive and negative subjective experiences share a common or distinct etiology.
The current report presents results of a study of retrospectively reported initial subjective experiences to nicotine exposure in a genetically informative sample. Subjective experiences to initial cigarette smoking were measured using the ESE during young adulthood. Our analyses were designed to investigate two questions. First, what were the magnitudes of genetic and environmental influences on subjective experiences? Second, what was the extent to which positive and negative experiences have a distinct or common underlying etiology?
Methods
Subjects
Siblings for the current study were drawn from the genetically informative sub-sample of the National Longitudinal Study of Adolescent Health (Add Health) [24]. Though followed longitudinally since 1992 (Wave I), initial subjective experiences were only collected in 2002 (Wave III). In 2002, 50.3% of the sibling-pairs sample was male, the mean age was 22.4 (± 1.75) years old. Participation rates were above 87% [24]. The majority (66.9%) self-reported Caucasian ethnicity, with 24.1% reporting Black and 8.9% either Asian or Native American. At Wave III, initial subjective experiences were collected from 352 same-sex monozygotic (MZ) twins (M: 171, F: 181) and 280 dizygotic (DZ) twins (M: 152, F: 128), 729 full-siblings (FS; M 355, F: 374), and 198 half-siblings (HS; M: 97, F: 101). Responses were also collected from opposite-sex (OS) siblings and totaled 923 (OSDZ: 213, OSFS: 528, OSHS: 182). Zygosity status of MZ and DZ twins was initially determined by self-report at Wave I and at Wave III using molecular markers.
Assessment
Subjective experiences were collected retrospectively using the nine-item Early Smoking Experience Questionnaire (ESE) [9]. Of these nine items, three (a pleasurable rush or buzz, relaxation, and pleasant sensations) assess positive experiences (POSITIVE; internal reliability, α, = .777) and five (unpleasant sensations, difficulty inhaling, nausea, heart pounding, and coughing) assess negative experiences (NEGATIVE; α = .823). Following on [9], dizziness was assessed and included in an overall nine-item hedonic measure of subjective experiences (HEDONIC; α = .776). Responses on the ESE are based on a 4-point Likert scale and could range from none (0) to intense (3). Item responses were dichotomized as being present or absent (1/0) and then summed within scale to create a total scale score. Total scores on the POSITIVE and NEGATIVE scales could therefore range from 0 to 3 and 0 to 5, respectively. Total scores on the HEDONIC scale could range from 0 to 9.
Statistical analyses
Sample characteristics were calculated using SAS/STAT software [25]. Polychoric correlations for the HEDONIC, POSITIVE, and NEGATIVE scales were determined using the statistical software Mx [26]. We utilized a liability threshold model to estimate the genetic and environmental influence on each of the three derived subjective effects scales. This model assumes an underlying normally liability trait, with the number of thresholds corresponding to the number of categories.
Univariate Analyses
Observed variation in data collected from same- and opposite-sex siblings can be parsed into their genetic and environmental components. Genetic influences includes additive [A] and non-additive [D] contributions while environmental influences include experiences shared [C] among siblings and those that are individual-specific [E]. In a purely genetic model, the MZ twin correlation would be twice that of DZ twin and FS sibling correlations, which themselves would twice that of the HS correlation. Shared environmental influences are implicated when the DZ twin and FS correlations are greater than one-half that of the MZ twin correlation.
Models were fit to allow for the ordinal character of the subjective experience scales using the raw maximum-likelihood estimation option in the statistical program (Mx) [27]. The significance of model parameters was evaluated by a comparison of the twice log-likelihood (-2LL) for models with or without the parameters, with the difference distributed as a chi-square distribution and the degrees of freedom being equal to the difference between the number of parameters estimated. A non-significant difference chi-square between two models indicated that the parameters dropped from the more parsimonious model were not significantly different from zero. Models were accepted on the basis of the Akaike Information Criterion (AIC) [28] which indicates the extent that a given model offers the most parsimonious, but adequate, explanation of the data.
Multivariate Analyses
Based on prior evidence [9, 12] indicating that dizziness loaded on both positive and negative subjective experiences, we examined two models: (1) independent pathways and (2) common pathways. Unlike univariate genetic analysis that examines the latent influences on variation in a single variable, these two models decompose the pattern of covariation among three or more variables into genetic and environmental influences that are common as well as unique to the different measures. The independent and common pathways models differ, however, in their hypothesis in that the common pathways model tests whether the covariation of dizziness, positive and negative subjective experiences is due to a single latent or underlying sensitivity factor that is itself influenced by genes and environments. A more direct contribution of genes and environments to subjective experiences is hypothesized by the independent pathways model. As these models specify a particular structure to the latent genetic and environmental influences, we first tested their appropriateness by comparing their fit to that of a saturated model which is agnostic to the structure of genetic and environmental effects on the measured variables [27]. Each multivariate model was fit to raw data and thresholds freely estimated.
Results
In the sibling-pairs sample, a total of 2645 participants reported ever having tried a cigarette. Of those who reported trying a cigarette, 2116 (80.0%) went on to smoke an entire cigarette. The majority of participants reported smoking an entire cigarette by age 19 (92.6%), with the mean age of initiation for males of 15.4 (± 2.82) and 15.6 (± 2.88) for females. Two-thirds (66.8%) reported smoking at least one cigarette a day for 30 days at some point in their life, and 62.6% reported smoking within the prior 30 days of being interviewed at Wave III.
Levels of initial subjective experiences were determined for 2482 participants (93.8%) who indicated they had ever tried a cigarette. As shown in Table 1, approximately two-thirds of the sample retrospectively reported slight to no subjective experiences. Confirmatory factor analyses supported a two factor solution, with dizziness loading equally on both a positive and negative factor. Mean scores and phenotypic correlations for the three experience scales are shown in Supplementary Table 1. Cross-trait correlations between POSITIVE and NEGATIVE experiences and dizziness with each of these two scales, respectively, were 0.34 (p < .01), 0.55 (p < .01), and 0.71 (p < .01). Homogeneity tests of the means and variances indicated there were no significant differences between the sexes or by zygosity status.
Table 1.
Endorsement Rates (%) of Nine Retrospectively Reported Subjective Experiences to Initial Cigarette Use (N = 2482).
| Extent of Subjective Experience |
||||
|---|---|---|---|---|
| Item | None | Slight | Moderate | Intense |
| Pleasant sensations | 50 | 28 | 17 | 5 |
| Unpleasant sensations | 42 | 30 | 18 | 11 |
| Nausea | 64 | 24 | 8 | 4 |
| Relaxation | 47 | 26 | 22 | 6 |
| Dizziness | 50 | 29 | 14 | 6 |
| Buzz/Head Rush | 48 | 28 | 17 | 8 |
| Coughing | 40 | 36 | 15 | 10 |
| Difficulty inhaling | 57 | 26 | 10 | 7 |
| Heart pounding | 70 | 21 | 6 | 2 |
Model Fitting
In general, higher MZ twin than DZ twin or FS correlations indicate heritable influences. For the POSITIVE and HEDONIC scales and dizziness, heritable influences are suggested. Though, for the POSITIVE scale, this pattern was less clear as a lower than expected DZ twin and higher than expected FS correlation may indicate C or D contributions, respectively [Table 2]. This interpretation though is unclear as the DZ twin and HS correlations were not significantly different from zero. For NEGATIVE effects, shared environmental influences appear to be important as the MZ and DZ twin correlations were not significantly different from one another.
Table 2.
Correlations (95% Confidence Intervals) for Subjective Effects to Initial Cigarette Use.
| Hedonic | Positive | Negative | Dizziness | |
|---|---|---|---|---|
| MZ | .32 (.14 - .47) | .31 (.11 - .49) | .20 (.01 - .37) | .30 (.05 – 53) |
| DZ | .19 (.04 - .33) | .05 (-.13- .23) | .21 (.05 - .37) | .21 (-.02 - .43) |
| FS | .14 (.04 - .24) | .27 (.16 - .37) | .09 (-.01 - .19) | .16 (.01 - .30) |
| HS | .04 (-.16 - .23) | -.16 (-.36 - .05) | .13 (-.07 - .33) | .06 (-.21 - .32) |
Abbreviations: MZ, monozygotic twins; DZ, dizygotic twins; FS, full-siblings; HS, half-siblings.
Univariate models
The overall fit our baseline model for our HEDONIC scale which included latent A, C, D and E effects that differed for males and females and sex-limited genetic effects was -2LL = 11207.33 (df = 2465). For the POSITIVE and NEGATIVE scales, the overall fit was 6347.94 (df = 2368) and 8755.39 (df = 2457), respectively. For dizziness, the -2LL was =3377.41 (df = 2445).
Against these baseline models we compared the fit of two models that examined the evidence for sex differences in the genetic and environmental influences on subjective experiences. Sex-specific thresholds could not be equated for all scales. There was no evidence supporting the sex-limited genetic influences (a′) on subjective experiences. Moreover, results from models that equated latent A, E and either C or D influences also suggested that the magnitude of such contributions were the same in males and females [Table 3]. Subsequent models that dropped either A, C, D, or some combination of these were fit with factors equated across sex and sex-specific thresholds.
Table 3.
Results of Univariate Model Fitting for Subjective Experiences Scales.
| Model | A | C | D | E | Δχ2 | Δdf | P | AIC |
|---|---|---|---|---|---|---|---|---|
| HEDONIC | ||||||||
| ACDE † | 0.30 (0.02 to 0.46) | 0.03 (0.00 to 0.17) | 0.00 (0.00 to 0.06) | 0.67 (0.54 to 0.84) | 3.80 | 5 | 0.58 | -6.20 |
| ACE | 0.30 (0.02 to 0.46) | 0.03 (0.00 to 0.17) | … | 0.67 (0.54 to 0.84) | 3.80 | 6 | 0.70 | -6.20 |
| ADE | 0.32 (0.21 to 0.46) | … | 0.00 (0.00 to 0.06) | 0.66 (0.54 to 0.87) | 3.95 | 6 | 0.68 | -8.05 |
| AE ‡ | 0.34 (0.22 to 0.46) | … | … | 0.66 (0.54 to 0.78) | 3.95 | 7 | 0.79 | -10.05 |
| CE | … | 0.17 (0.10 to 0.23) | … | 0.83 (0.77 to 0.90) | 8.30 | 7 | 0.31 | -5.70 |
| POSITIVE | ||||||||
| ACDE † | 0.32 (0.00 to 0.53) | 0.02 (0.00 to 0.25) | 0.08 (0.00 to 0.19) | 0.59 (0.42 to 0.83) | 1.33 | 5 | 0.93 | -8.67 |
| ACE | 0.38 (0.00 to 0.56) | 0.01 (0.00 to 0.20) | … | 0.69 (0.44 to 0.86) | 2.74 | 6 | 0.84 | -9.26 |
| ADE | 0.35 (0.16 to 0.53) | … | 0.08 (0.08 to 0.19) | 0.57 (0.41 to 0.75) | 1.37 | 6 | 0.97 | -10.63 |
| AE ‡ | 0.40 (0.22 to 0.56) | … | … | 0.60 (0.44 to 0.78) | 0.91 | 7 | 0.91 | -11.26 |
| CE | … | 0.17 (0.08 to 0.25) | … | 0.83 (0.75 to 0.91) | 6.14 | 7 | 0.52 | -7.86 |
| NEGATIVE | ||||||||
| ACDE † | 0.13 (0.00 to 0.36) | 0.08 (0.00 to 0.20) | 0.00 (0.00 to 0.08) | 0.80 (0.64 to 0.93) | 3.80 | 5 | 0.58 | -6.20 |
| ACE ‡ | 0.13 (0.00 to 0.36) | 0.08 (0.08 to 0.20) | … | 0.80 (0.64 to 0.93) | 1.55 | 6 | 0.96 | -10.45 |
| ADE | 0.25 (0.11 to 0.38) | … | 0.00 (0.00 to 0.07) | 0.75 (0.62 to 0.89) | 2.47 | 6 | 0.87 | -9.53 |
| AE | 0.25 (0.11 to 0.38) | … | … | 0.75 (0.62 to 0.89) | 2.47 | 7 | 0.93 | -11.53 |
| CE | … | 0.14 (0.06 to 0.21) | … | 0.86 (0.79 to 0.94) | 2.29 | 7 | 0.94 | -11.71 |
| DIZZINESS | ||||||||
| ACDE† | 0.29 (0.00 to 0.52) | 0.04 (0.00 to 0.24) | 0.00 (0.00 to 0.53) | 0.68 (0.45 to 0.90) | 3.80 | 5 | 0.58 | -6.20 |
| ACE | 0.29 (0.00 to 0.52) | 0.04 (0.00 to 0.24) | … | 0.68 (0.48 to 0.90) | 2.16 | 6 | 0.90 | -9.84 |
| ADE | 0.34 (0.02 to 0.52) | … | 0.00 (0.00 to 0.53) | 0.66 (0.44 to 0.66) | 2.27 | 6 | 0.86 | -9.74 |
| AE ‡ | 0.34 (0.15 to 0.52) | … | … | 0.66 (0.48 to 0.85) | 2.27 | 7 | 0.94 | -11.74 |
| CE | … | 0.17 (0.07 to 0.27) | … | 0.83 (0.73 to 0.93) | 4.16 | 7 | 0.76 | -9.84 |
Abbreviations: AIC, Akaike Information Criteria; A, additive genetic influences; C, shared environmental influences; D, non-additive genetic influences; E, non-shared environmental influences.
Best-fitting model.
Allowing different prevalence for males and females, latent A, C, D and E factors equated across sex, no sex-limited (a′) genetic influences.
As judged by AIC, dropping D from our baseline model resulted in an improvement in model fit for all scales [Table 3]. For dizziness, HEDNOIC, and POSITIVE scales, dropping C also resulted in an improvement in model fit. For the NEGATIVE scale, both an AE and CE model resulted in similar improvements in model fit and were therefore inconclusive. Accordingly, we selected an ACE model as most parsimonious explanation for the observed variation in NEGATVE experiences. Across the three scales, genetic influences were small to moderate and ranged from 0.13 for NEGATIVE experiences to 0.40 for POSITIVE experiences. Individual-specific environmental contributions were substantial for all three scales.
Multivariate Models
We first compared the fit of independent and common pathways models that specified A, C, and E influences against a saturated model with an unspecified latent genetic and environmental factor structure. Model fit results indicated that both the independent (χ2 = 116.61, Δdf = 108, p = 0.31, AIC = -103.39) and common (χ2 = 122.75, Δdf = 112, p = 0.23, AIC = -101.25) pathways models provided a better fit.
Results for the common pathways model are presented in Figure 2. A model including latent A, C, and E factors is shown. Against this model, we next tested whether the genetic and environmental influences on the covariation of diverse subjective experiences act more directly on drug actions. When the fit of these two models were compared, the difference was found to be non-significant (Δχ2 = 8.21, Δdf = 4, p = 0.08). This indicated that the common pathways model provided a more parsimonious but adequate fit to these data than the independent pathways model. Accordingly, the common pathways model suggested that multiple genes and environments, each of small effect, contribute to a latent sensitivity that in turn influences the covariation of diverse subjective experiences. Testing nested sub-models that dropped paths whose confidence intervals included zero further supported this observation. Of these, models that dropped C influences on the latent phenotype (Δχ2 = 0.00, Δdf = 1, p = 0.99, AIC = -2.00), residual C influences (Δχ2 = 1.55, Δdf = 3, p = 0.67, AIC = -4.45), and subsequently together (Δχ2 = 1.55, Δdf = 4, p = 0.82, AIC = -6.45), all resulted in an improvement in model fit. Furthermore, residual A influences on all but positive experiences could be dropped (Δχ2 = 3.22, Δdf = 6, p = 0.78, AIC = -8.78). This final model indicated that the genetic and environmental effects on dizziness are primarily those that also influence positive and negative experiences.
Figure 2.
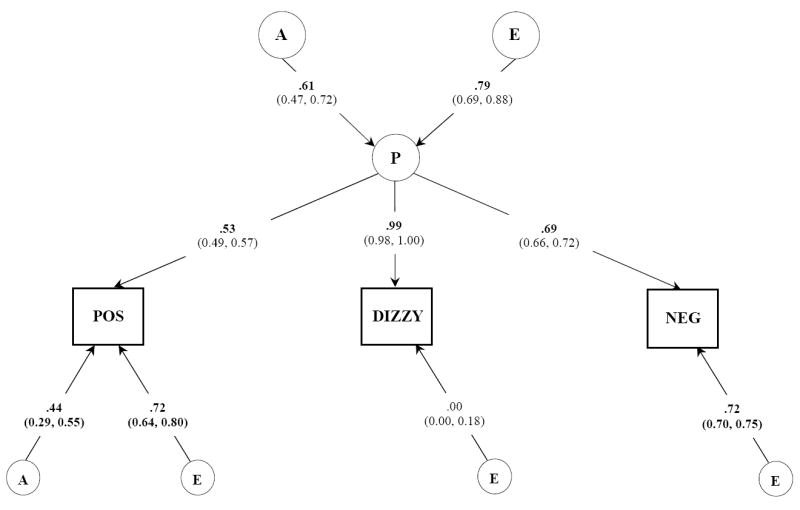
Best fitting common pathways model. Unstandardized parameter estimates (95% confidence intervals) are shown. A, indicates additive genetic influences; C, indicates shared environment; E, indicates individual specific environmental experiences and includes measurement error. P, common underlying pathway or phenotype; POS, positive subjective experiences; NEG, negative subjective experiences.
The resulting most parsimonious model of the genetic and environmental contributions to the covariation of dizziness, positive and negative experiences and standardized parameter estimates are shown in Figure 3. Parameter estimates are similar to those obtained from our univariate models and indicate that our multivariate analyses had sufficient power.
Discussion
In this genetically informative sample of young adults, we sought to determine the extent that heritable factors contributed to variation in subjective experiences to initial cigarette use and whether the covariation of diverse subjective experiences was due to genetic and environmental influences that impacted an overall sensitivity to the autonomic and sensory effects of initial cigarette use.
Many aspects of cigarette use are influenced by genetic and environmental influences. Cigarette initiation, in general, is influence by shared and individual specific environmental influences [29-31]. In contrast, nicotine dependence is strongly influenced by genetic factors and small individual specific environmental experiences [30, 32-33]. Progression to and regular use of cigarettes evidence small to moderate genetic effects and no shared environmental influences, with estimates varying as a function of the sample and age group examined [33-36]. Together these and other studies support the notion that early environmental experiences are important factor to initiating cigarette use, but that continued and problematic use are influenced by heritable factors. That the genetic influences on many later smoking behaviors are highly correlated [37-39] further elaborates this observation.
In our univariate analyses, we examined, to our knowledge for the first time, the extent genetic influences contributed to variation in the subjective experiences of initial cigarette use and whether they differed between males and females. By comparison with simpler models, we did not find support for sex-limited genetic effects or that the magnitude of heritable and environmental influences differed for males and females. These observations extend studies of smoking initiation and raises the possibility that magnitude differences observed for more problematic smoking behaviors [31, 35, 40-42] may emerge after regular and persistent use has developed. Our results also suggest that genetic factors contributed moderately to individual differences in dizziness, positive and the overall hedonic experiences of initial cigarette use. Given the salience of these experiences in retrospective reports by adult and adolescent smokers [18, 23], the role they have in theories of nicotine dependence [2, 3, 5, 6], and the heritable contributions to different smoking behaviors [29-39], our results raise the possibility that the genetic influences on these initial experiences may also be those that are involved in regular and problematic cigarette use. This observation is consistent with evidence from molecular genetic studies of initial experiences [43- 46], smoking initiation and progression [47], as well as nicotine dependence [48, 49].
Although genetic influences are meaningful factors in the individual differences that contribute to how cigarettes are initially experienced, our results also underscore the importance of environmental influences. This observation is particularly relevant to negative experiences for which heritable effects were small and shared environmental influences could not be excluded. In general, however, individual specific environmental experiences, which include measurement error, appear to be the most salient. As such, intervention efforts that target those experiences unique to a siblings such as self-efficacy and other personal perceptions, peer influences, and risk perceptions [50-58] on the basis of how they experienced their initial cigarette use may provide a useful approach to tobacco control. Similarly, although mixed, evidence does suggest that negative experiences may be an important indicator of a later vulnerability [9, 18, 19]. As such, interventions that address those environmental influences shared equally between siblings such as parental smoking history or general smoking environment [52, 53, 58] may also be an effective strategy.
The second question we sought to answer concerned the structure of genetic and environmental influences on the covariation of diverse subjective experiences. Using two multivariate models, results suggested that multiple genetic [e.g. additive] and environmental factors contributed to an underlying sensitivity which influenced the subjective experiences to initial cigarette use. In the best-fitting model, this latent sensitivity was moderately heritable and evidenced strong individual-specific environmental influences. Further, residual genetic influences were identified only for positive experiences, indicating that additional genetic factors are influencing these types of experiences to initial cigarette use, over and above the common influence of a general sensitivity to the chemicals contained in a cigarette.
The latent general sensitivity to cigarette exposure assessed by the ESE loaded most strongly on dizziness and moderately on positive and negative experiences. This indicates that differences in initial subjective experiences are more directly related dizziness than either positive or negative experiences. It also suggests that individual differences in positive and negative experiences are in part due to heritable and environmental influences on dizziness. This shared etiology extends the common observation that both positive and negative experiences are endorsed together by the same respondent. Further, given the predictive utility of dizziness [9, 20, 21] and of pleasant, but not unpleasant, dizziness [59], these two experiences together may provide additional clinical utility in the identification of those at risk for later problematic cigarette use as well as aid the identification of the heritable factors involved. Lastly, given the absence of residual genetic effects, these results suggest that dizziness may be the most genetically informative subjective experience.
Similar to our univariate results, our best-fitting multivariate model underscored the importance of environmental experiences unique to siblings. While all three experiences examined here have some similar environmental influences, our results suggest that the bulk of individual specific environmental influences are largely experience specific. For negative effects, in particular, this suggests that that they have a more complex etiology than positive experiences. Further, they indicate that attempts to identify relevant environmental influences on subjective effects need to differentiate between those that broadly impact subjective experiences and those which influence positive and negative separately.
Our findings should be considered in the context of a number of limitations. First, subjective experiences were retrospectively reported. As much of our sample continued using cigarettes after their initial few, subsequent exposure to the autonomic and sensory effects of cigarettes may bias the recall of initial experiences. Second, the sizeable impact of individual specific environments, which includes measurement error, suggests that some experiences or sets of experiences may be better recalled than others. This would reduce the ability to detect genetic contributions and may influence the patterns of covariation. Finally, natural differences in ‘puff topography’ or the depth and length of inhalation, number of puffs, and quality of the cigarette smoked [60] may have contributed to our results. Though adolescents deliver a pharmacologically active dose of nicotine [2], such features of ‘puff topography’ were not collected and could thus remain a potential source of variation.
Supplementary Material
Figure 1.
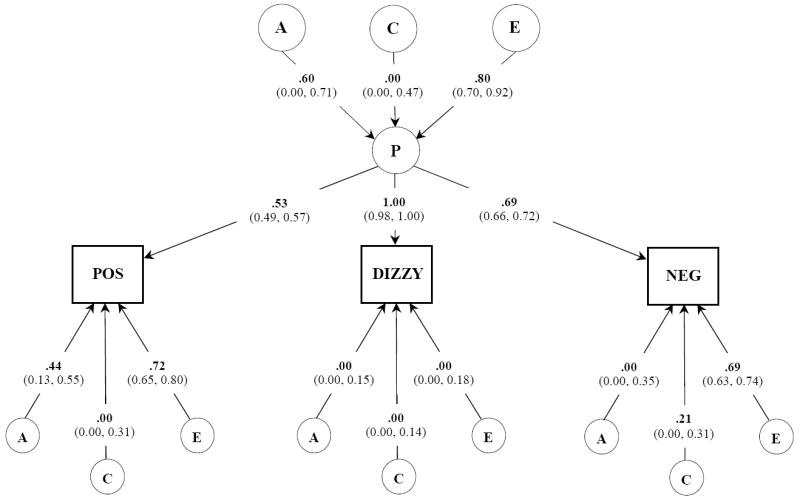
Common pathways model. Unstandardized parameter estimates (95% confidence intervals) are shown. The chi-square goodness-of-fit for the overall ACE model is: -2LL = 17712.86, d f= 7470. Circles depict latent variables while rectangles depict self-reported subjective experiences. Single headed arrows represent the partial regression of an observed variable on the latent factor. Variances for each observed variable was standardized to 1.0. A, indicates additive genetic influences; C, indicates shared environmental influences; E, indicates individual specific environmental experiences; P, common underlying pathway or phenotype; POS, positive subjective experiences; NEG, negative subjective experiences.
Acknowledgments
This research uses data from Add Health, a program project designed by J. Richard Udry, Peter S. Bearman, and Kathleen Mullan Harris, and funded by a grant P01-HD31921 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, with cooperative funding from 17 other agencies. Special acknowledgement is due Ronald R. Rindfuss and Barbara Entwisle for assistance in the original design. Persons interested in obtaining data files from Add Health should contract Add Health, Carolina Population Center, 123 W. Franklin Street, Chapel Hill, NC, 275-2524 (addhealth@unc.edu). BCH, JKH and CJH were supported by grant R01-HD031921. Additional support for CJH and MAE was through grant R01-DA021913 and R01-AA017889, respectively.
Footnotes
Conflict of interest statement: The authors declare no conflict of interest.
Literature Cited
- 1.Tercyak KP, Rodiguez D, Audrain-McGovern J. High school seniors’ smoking initiation and progression 1 year after graduation. American Journal of Public Health. 2007;97:1397–1398. doi: 10.2105/AJPH.2006.094235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eissenberg T, Balster RL. Initial tobacco use episodes in children and adolescents: current knowledge, future directsion. Drug and Alcohol Dependence. 2000;59:S41–S60. doi: 10.1016/s0376-8716(99)00164-7. [DOI] [PubMed] [Google Scholar]
- 3.Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nature Neuroscience. 2005;8:1481–1490. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- 4.Hyman SE, Malenka RC. Addiction and the brain: the neurobiology of compulsion and its persistence. Nature Reviews Neuroscience. 2001;2:965–703. doi: 10.1038/35094560. [DOI] [PubMed] [Google Scholar]
- 5.Glautier S. Measures and models of nicotine dependence: positive reinforcement. Addiction. 2004;1:30–50. doi: 10.1111/j.1360-0443.2004.00736.x. [DOI] [PubMed] [Google Scholar]
- 6.Pomerleau OF. Individual differences in sensitivity to nicotine: Implications for genetic research on nicotine dependence. Behavior Genetics. 1995;25:161–178. doi: 10.1007/BF02196925. [DOI] [PubMed] [Google Scholar]
- 7.Lambert NM, McLeod M, Schenk S. Subjective responses to initial experience with cocaine: an exploration of the incentive-sensitization theory of drug abuse. Addiction. 2006;101:713–725. doi: 10.1111/j.1360-0443.2006.01408.x. [DOI] [PubMed] [Google Scholar]
- 8.Kalman D. The subjective effects of nicotine: methodological issues, a review of experimental studies, and recommendations for future research. Nicotine & Tobacco Research. 2002;4:25–70. doi: 10.1080/14622200110098437. [DOI] [PubMed] [Google Scholar]
- 9.Pomerleau OF, Pomerleau CS, Namenek RJ. Early experiences with tobacco among women smokers, ex-smokers, and never-smokers. Addiction. 1998;93:595–599. doi: 10.1046/j.1360-0443.1998.93459515.x. [DOI] [PubMed] [Google Scholar]
- 10.Lyons MJ, Toomey R, Meyer JM, Green AI, Eisen SA, Goldberg J, et al. How do genes influence marijuana use? The role of subjective effects. Addiction. 1997;92:409–417. [PubMed] [Google Scholar]
- 11.Perkins KA, Jetton C, Keenan J. Common factors across acute subjective effects of nicotine. Nicotine & Tobacco Research. 2003;6:869–875. doi: 10.1080/14622200310001614629. [DOI] [PubMed] [Google Scholar]
- 12.Rodriguez D, Audrian-McGovern J. Construct validity analysis of the early smoking experience questionnaire for adolescents. Addictive Behaviors. 2004;29:1053–1057. doi: 10.1016/j.addbeh.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 13.Kandel DB, Hu MC, Griesler PC, Schaffran C. On the development of nicotine dependence in adolescence. Drug & Alcohol Dependence. 2007;91:26–39. doi: 10.1016/j.drugalcdep.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen X, Stacy A, Zheng H, Shan J, Spruijt-Metz D, Unger JB, et al. Sensations from initial exposure to nicotine predicting adolescent smoking in China: A potential measure of vulnerability to nicotine. Nicotine & Tobacco Research. 2003;5:455–463. doi: 10.1080/14622200307239. [DOI] [PubMed] [Google Scholar]
- 15.Riedel BW, Blitstein JL, Robinson LA, Murray DM, Klesges RC. The reliability and predictive value of adolescents’ reports of initial reactions to smoking. Nicotine & Tobacco Research. 2003;5:553–559. doi: 10.1080/1462220031000118658. [DOI] [PubMed] [Google Scholar]
- 16.DiFranza JR, Savageau JA, Fletcher K, Pbert L, O’Loughlin J, McNeill AD, et al. Susceptiblity to nicotine dependence: The development and assessment of nicotine dependence in Youth 2 Study. Pediatrics. 2007;120:e974–e983. doi: 10.1542/peds.2007-0027. [DOI] [PubMed] [Google Scholar]
- 17.DiFranza JR, Savageau JA, Fletcher K, Ockene JK, Rigotti NA, McNeill AD, et al. Recollections and repercussions of the first inhaled cigarette. Addictive Behaviors. 2004;29:261–272. doi: 10.1016/j.addbeh.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 18.Hu MC, Davies M, Kandel DB. Epidemiology and correlates of daily smoking and nicotine dependence among young adults in the United States. American Journal of Public Health. 2006;96:299–308. doi: 10.2105/AJPH.2004.057232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pomerleau OF, Pomerleau CS, Mehringer AM, Snedecor SM, Cameron OG. Validation of retrospective reports of early experiences with smoking. Addictive Behaviors. 2005;30:607–611. doi: 10.1016/j.addbeh.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 20.Pomerleau CS, Pomerleau OF, Namenek RJ, Marks JL. Initial exposure to nicotine in college-age women smokers and never-smokers: a replication and extension. Journal of Addictive Diseases. 1999;18:13–19. doi: 10.1300/J069v18n03_02. [DOI] [PubMed] [Google Scholar]
- 21.Perkins KA, Lerman C, Coddington S, Karelitz JL. Association of retrospective early smoking experiences with prospective sensitivity to nicotine via nasal spray in nonsmokers. Nicotine & Tobacco Research. 2008;8:1335–1345. doi: 10.1080/14622200802238886. [DOI] [PubMed] [Google Scholar]
- 22.Hirschman RS, Leventhal H, Glynn K. The development of smoking behavior: conceptualization and supportive cross-sectional survey data. Journal of Applied Social Psychology. 1984;14:184–206. [Google Scholar]
- 23.Pomerleau OF, Pomerleau CS, Snedecor SM, Gaulrapp S, Kardia SLR. Heterogeneity in phenotypes based on smoking status in the Great Lakes Smoker Sibling Registry. Addictive Behaviors. 2004;30:607–611. doi: 10.1016/j.addbeh.2004.03.036. [DOI] [PubMed] [Google Scholar]
- 24.Harris MH, Halpern CT, Smolen A, Haberstick BC. The National Longitudinal Study of Adolescent Health (Add Health) twin data. Twin Research and Human Genetics. 2006;9:988–997. doi: 10.1375/183242706779462787. [DOI] [PubMed] [Google Scholar]
- 25.Version 9 of the SAS system for Linux. Copyright © 2002-2003 by SAS Institute Inc; Cary, North Carolina, USA: [Google Scholar]
- 26.Neale MC, Boker SM, Xie G, Maes H. Mx: Statistical Modeling. Box 126 MCV, Richmond, VA 23298: Department of Psychiatry; 1999. [Google Scholar]
- 27.Neale MC, Cardon LR. Methodology for Genetic Study of Twins and Families. Dordrecht: Kluwer Academic Publishers; 1992. [Google Scholar]
- 28.Akaike H. Factor analyses and AIC. Psychometrika. 1987;52:317–322. [Google Scholar]
- 29.Fowler T, Lifford K, Shelton K, Rice F, Thapar A, Neale MC, et al. Exploring the relationship between genetic and environmental influences on initiation and progression of substance use. Addiction. 2007;101:413–422. doi: 10.1111/j.1360-0443.2006.01694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vink JM, Willemsen G, Boomsma DI. Heritability of smoking initiation and nicotine dependence. Behavior Genetics. 2005;35:397–406. doi: 10.1007/s10519-004-1327-8. [DOI] [PubMed] [Google Scholar]
- 31.Rhee SH, Hewitt JK, Young SE, Corley RP, Crowley TJ, Stallings MC. Genetic and environmental influences on substance initiation, use, and problem use in adolescents. Archives of General Psychiatry. 2003;60:1256–1265. doi: 10.1001/archpsyc.60.12.1256. [DOI] [PubMed] [Google Scholar]
- 32.Haberstick BC, Timberlake D, Ehringer MA, Lessem JM, Hopfer CJ, Smolen A, Hewitt JK. Genes, time to first cigarette and nicotine dependence in a general population sample of young adults. Addiction. 2007;102:655–665. doi: 10.1111/j.1360-0443.2007.01746.x. [DOI] [PubMed] [Google Scholar]
- 33.Lessov CN, Martin NG, Statham DJ, Todorov AA, Slutske WS, Bucholz KK, et al. Defining nicotine dependence for genetic research: evidence from Australian twins. Psychological Medicine. 2004;34:1–15. doi: 10.1017/s0033291703001582. [DOI] [PubMed] [Google Scholar]
- 34.Stallings MC, Hewitt JK, Beresford T, Heath AC, Eaves LJ. A twin study of drinking and smoking onset and latencies from first use to regular use. Behavior Genetics. 1999;29:409–421. doi: 10.1023/a:1021622820644. [DOI] [PubMed] [Google Scholar]
- 35.Koopmans JR, Slutske WS, Heath AC, Neale MC, Boomsma DI. The genetics of smoking initiation and quantity smoked in Dutch adolescents and young adult twins. Behavior Genetics. 1999;29:383–393. doi: 10.1023/a:1021618719735. [DOI] [PubMed] [Google Scholar]
- 36.Li MD, Cheng R, Ma JZ, Swan GE. A meta-analysis of estimated genetic and environmental effects on smoking behavior in male and female adult twins. Addiction. 2003;98:23–31. doi: 10.1046/j.1360-0443.2003.00295.x. [DOI] [PubMed] [Google Scholar]
- 37.Kendler KS, Neale MC, Sullivan P, Corey LA, Gardner CO, Prescott CA. A population-based twin study in women of smoking initiation and nicotine dependence. Psychological Medicine. 1999;29:299–308. doi: 10.1017/s0033291798008022. [DOI] [PubMed] [Google Scholar]
- 38.Maes HH, Sullivan PF, Bulik CM, Neale MC, Prescott CA, Eaves LJ, et al. A twin study of genetic and environmental influences on tobacco initiation, regular tobacco use and nicotine dependence. Psychological Medicine. 2004;34:1–11. doi: 10.1017/s0033291704002405. [DOI] [PubMed] [Google Scholar]
- 39.Pergadia ML, Heath AC, Martin NG, Madden PAF. Genetic analysis of DSM-IV nicotine withdrawal in adult twins. Psychological Medicine. 2006;36:963–972. doi: 10.1017/S0033291706007495. [DOI] [PubMed] [Google Scholar]
- 40.Madden PA, Pedersen NL, Kaprio J, Koskenvuo MJ, Martin NG. The epidemiology and genetics of smoking initiation and persistence: cross-cultural comparisons of twin study results. Twin Research. 2004;7:82–97. doi: 10.1375/13690520460741471. [DOI] [PubMed] [Google Scholar]
- 41.Heath AC, Martin NG. Genetic models of the natural history of smoking: evidence for a genetic influence on smoking persistence. Addictive Behaviors. 1993;18:19–34. doi: 10.1016/0306-4603(93)90005-t. [DOI] [PubMed] [Google Scholar]
- 42.Han C, McGue MK, Iacono WG. Lifetime tobacco, alcohol and other substance use in adolescent Minnesota twins: univariate and multivariate behavioral genetic analyses. Addiction. 1999;94:981–993. doi: 10.1046/j.1360-0443.1999.9479814.x. [DOI] [PubMed] [Google Scholar]
- 43.Sherva R, Wilhelmsen K, Pomerleau CS, Chasse SA, Rice JP, Snedecor SM, et al. Association of a single nucleotide polymorphism in neuronal acetylcholine receptor subunit alpha 5 (CHRNA5) with smoking status and with ‘pleasurable buzz’ during early experimentation with smoking. Addiction. 2008;103:1544–1552. doi: 10.1111/j.1360-0443.2008.02279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ehringer MA, Clegg HV, Collins AC, Corely RP, Crowley R, Hewitt JK, et al. Association of the neuronal nicotinic receptor beta2 subunit (CHRNB2) with subjective responses to alcohol and nicotine. American Journal of Medical Genetics. 2007;144B:596–604. doi: 10.1002/ajmg.b.30464. [DOI] [PubMed] [Google Scholar]
- 45.Zeiger JS, Haberstick BC, Schlaepfer I, Collins AC, Corley RP, Crowley T, et al. The neuronal nicotinic receptor subunit genes (CHRNA6 and CHRNB3) are associated with subjective response to tobacco. Human Molecular Genetics. 2008;17:724–734. doi: 10.1093/hmg/ddm344. [DOI] [PubMed] [Google Scholar]
- 46.Hutchison KE, Allen DL, Filbey FM, Jepson C, Lerman C, Benowtiz NL, et al. CHRNA4 and tobacco dependence: from gene regulation to treatment outcome. Archives of General Psychiatry. 2007;64:1078–1086. doi: 10.1001/archpsyc.64.9.1078. [DOI] [PubMed] [Google Scholar]
- 47.Wang J, Li MD. Common and unique biological pathways associated with smoking initiation/progression, nicotine dependence, and smoking cessation. Neuropsychopharmacology. 2010;35:702–719. doi: 10.1038/npp.2009.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bierut LJ, Madden PA, Breslau N, Johnson EO, Hatsukami D, Pomerleau OF, et al. Novels genes identified in a high-density genome wide association study for nicotine dependence. Human Molecular Genetics. 2007;16:24–35. doi: 10.1093/hmg/ddl441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saccone SF, Hinrichs AL, Saccone NL, Chase GA, Konvicka K, Madden PA, et al. Cholinergic nicotinic receptor genes implicated in a nicotine dependence association study targeting 348 candidate genes with 3713 SNPs. Human Molecular Genetics. 2007;16:36–49. doi: 10.1093/hmg/ddl438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ausems M, Mesters I, van Breukelen G, de Vries H. Do Dutch 11-12 years olds who never smoke, smoke experimentally or smoke regularly have different demographic backgrounds and perceptions of smoking? European Journal of Public Health. 2003;13:160–167. doi: 10.1093/eurpub/13.2.160. [DOI] [PubMed] [Google Scholar]
- 51.Wang MQ, Eddy JM, Fitzhugh EC. Smoking acquisition: peer influence and self-selection. Psychological Reports. 2000;86:1241–1246. doi: 10.2466/pr0.2000.86.3c.1241. [DOI] [PubMed] [Google Scholar]
- 52.Okoli CTC, Richardson CG, Johnson JL. An examination of the relationship between adolescents’ initial smoking experience and their exposure to peer and family member smoking. Addictive Behaviors. 2008;33:1183–1191. doi: 10.1016/j.addbeh.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 53.Gilman SE, Rende R, Boergers J, Abrams DB, Buka SL, Clark MA, et al. Parental smoking and adolescent smoking initiation: An intergenerational perspective on tobacco control. Pediatrics. 2009;123:e274–e281. doi: 10.1542/peds.2008-2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kaufman AR, Augustson EM. Predictors of regular cigarette smoking among adolescent females: does body image matter? Nicotine & Tobacco Research. 2008;10:1301–1309. doi: 10.1080/14622200802238985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McLeod K, White V, Mullins R, Davey C, Wakefield M, Hill D. How do friends influence smoking uptake? Findings from qualitative interviews with identical twins. Journal of Genetic Psychology. 2008;169:117–131. doi: 10.3200/GNTP.169.2.117-132. [DOI] [PubMed] [Google Scholar]
- 56.Tomeo CA, Field AE, Berkey CS, Colditz GA, Frazier AL. Weight concerns, weight control behaviors, and smoking initiation. Pediatrics. 1999;104:918–924. doi: 10.1542/peds.104.4.918. [DOI] [PubMed] [Google Scholar]
- 57.Nichols TR, Birnbaum AS, Birnel S, Botvin GJ. Perceived smoking environment and smoking initiation among multi-ethnic urban girls. Journal of Adolescent Health. 2006;38:369–375. doi: 10.1016/j.jadohealth.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 58.Milton B, Woods SE, Dugdill L, Porcellato L, Springett RJ. Starting young? Children’s experiences of trying smoking during pre-adolescence. Health Education Research. 2008;23:298–309. doi: 10.1093/her/cym027. [DOI] [PubMed] [Google Scholar]
- 59.Kandel DB, Hu MC, Griesler P, Schaffran C. On the development of nicotine dependence in adolescence. Drug and Alcohol Dependence. 2007;91:26–39. doi: 10.1016/j.drugalcdep.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brady SS, Song AV, Halpern-Felsher BL. Adolescents report both positive and negative consequences of experimentation with cigarette use. Preventative Medicine. 2008;46:585–590. doi: 10.1016/j.ypmed.2008.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


