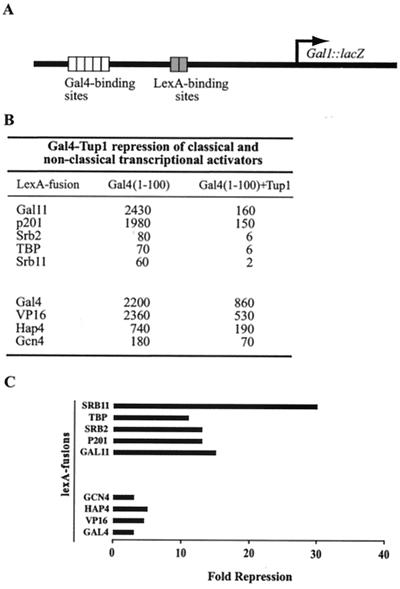
Figure 1.
Tup1 represses transcription mediated by nonclassical activators particularly efficiently. (A) The reporter used in the repression assays contains five Gal4 DNA-binding sites upstream of two lexA sites which are, in turn, positioned 50 bp upstream from the natural GAL1 TATA box. (B and C) Cells harboring the reporter gene were cotransformed with plasmids expressing Gal4-Tup1 and a variety of LexA-fusion activators. In each case, LexA-fusion activators consisted of full-length protein with lexA replacing the respective DNA-binding domain where applicable. Gal4-Tup1 consisted of Gal4 (residues 1–100) fused to full-length Tup1. Cells were assayed for GAL1-lacZ expression by β-galactosidase activity (B). The numbers represent the average of assays on three independent colonies performed in duplicate. The standard errors were typically 10–15%. The fold repression, shown in C, was calculated as the ratio of values obtained with and without the repressor function of Tup1.