Figure 3.
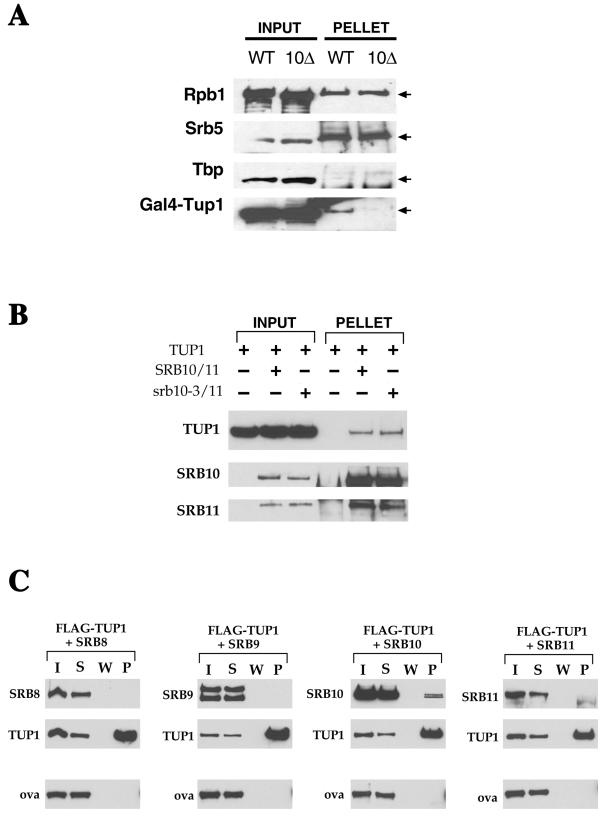
Tup1 interacts with the holoenzyme, at least in part, through its interactions with Srb10. (A) Tup1 immunoprecipitates with the holoenzyme found in wild-type extracts but much less so with holoenzyme found in extracts from a strain deleted for Srb10. Purified Tup1 was incubated with equivalent amounts of nuclear extracts of either the wild-type strain or an isogenic strain lacking Srb10 (Input). Each extract contained equivalent levels of Rpb1 (largest subunit of the RNA polymerase II), Srb5 (an integral component of the holoenzyme), and Tbp (the TATA binding protein which does not stably associate with the RNA pol II holoenzyme). Holoenzyme and proteins bound to it were precipitated with affinity-purified antibodies against-Srb5 (Pellet). As expected, Tbp did not immunoprecipitate well, whereas both Srb5 and Rpb1 did immunoprecipitate efficiently. (B) Tup1 interacts with Srb10/11 complex. Purified Tup1 was incubated with purified Srb10/11 complex (Input) and then immunoprecipitated by using affinity-purified anti-Srb10 antibodies (Pellet). Srb10–3 has a point mutation in the kinase domain that eliminates the catalytic function of the enzyme without altering its ability to interact with its cyclin partner or the holoenzyme. (C) Tup1 interacts with Srb10. Purified, flag epitope-tagged Tup1 was incubated with four components of the repression subcomplex of the holoenzyme. Anti-flag monoclonal antibody was used to immunoprecipitate the tagged Tup1 incubated individually with insect-cell extracts containing overexpressed Srb8, 9, 10, and 11. The input (I), the supernatant (S), the wash (W), and the precipitated (P) levels of each protein in the reaction are shown. Ovalbumin was used as a control for nonspecific aggregation. In lane P of the Srb11 subpanel, the faster migrating band is not a degradation product of Srb11 but a chain of the flag monoclonal antibody that is detected by the secondary antibody used in the immunoblot.