Figure 7.
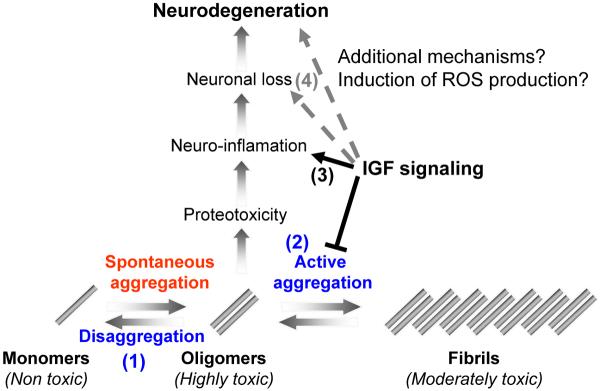
IGF-1 signaling can play several roles in mitigating the toxicity of Aβ. The digestion of APP creates Aβ monomers that spontaneously aggregate to form toxic oligomers and possibly higher order structures in-vivo. At least two biological mechanisms can detoxifiy Aβ oligomers: (1) conversion of toxic oligomers into monomers (disaggregation) and (2) the conversion of the toxic oligomers into a much less toxic, larger structures (active aggregation). Within scenario 1, IGF-1 signaling could normally funciton to reduce protein disaggregases, possibly mediated by HSF transcription factors. Therefore, reduction of IGF-1 signaling would be predicted to result in less oligomers and more monomeric forms of Aβ due to the activation of protein disaggregases. Our resutls are inconsistent with this scenario since we find less oligomers, but equal amounts of monomeric forms of Aβ. Alternatively, within in scenario 2, IGF-1 signaling could normally function to reduce protective protein aggregases that convert toxic species into larger, less toxic forms. Therefore, reduced IGF-1 signaling results in increased aggreagase activity that in turn reduces the load of toxic oligomers and increases the compaction of less toxic fibrils. In support of scenario 2, we observed less soluble oligomers and higher compaction of the amyloid plaques in the AD animals with reduced IGF1R signaling (AD;Igf1r+/− animals). Alternatively, (3) IGF-1 signaling could promote protoeotoxicity and neuro-inflamation in response to toxic Aβ assemblies. Our results are also consistent with this proposed mechanism as we observed much less neuro-inflammation in the brains of protected AD;Igf1r+/− animals. However, this lower inflammation rate could be directly related to the reduction of Aβ oligomers in the protected animals by increased aggregases. Finally,in scenario 4, reduction of toxic secondary factors, such as Reactive Oxygen Species (ROS), might synergize with the production of toxic Aβ assemblies to promote neuronal loss. Consistent with this mechanism, Igf1r+/− mice are much more resistant to oxidative damage than wild type mice. Taken together, IGF-1 signaling could impinge at multiple steps on the path to neuronal loss and neurodegeneration in response to Aβ production and none of the interventions are mutually exclusive. Therefore, methods to reduced IGF-1 signaling provide multiple opportunities for disease intervention. Our data is most consistent with a model in which reduced IGF-1 signaling reduces the load of toxic Aβ structures, presumably dimers, that results in higher compaction of plaques, reduced neruo-inflammation and reduced neuronal loss.