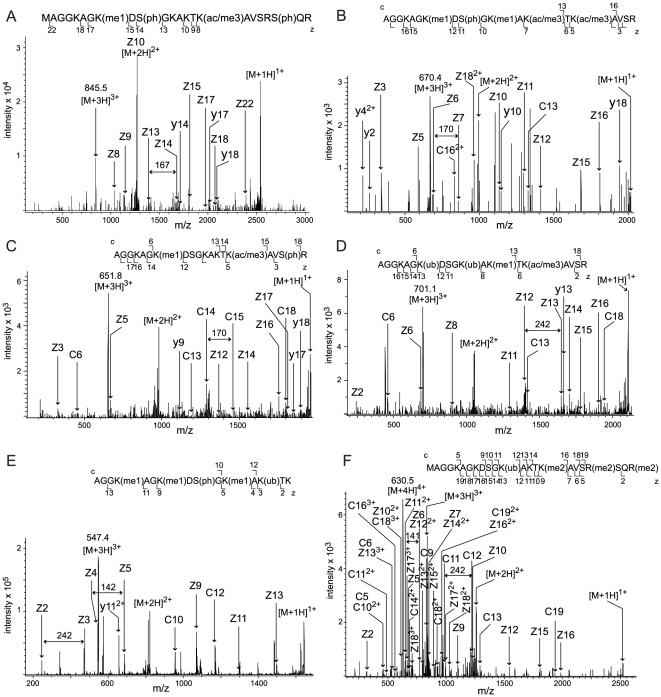
Figure 2. Differentially processed and modified N-termini of histone H2AFZ.
ETD fragmentation spectra corresponding to six differentially processed N-terminal peptides of the variant H2AFZ (GI: 4504255). A–E show peptides identified in the histone analysis optimization process, while F presents the spectrum of the peptide identified during the following nanoLC/MS/MS analysis of six different subjects. The positions of z,y (C-terminal) and c (N-terminal) fragment ions are indicated in the spectra and in the presented peptide sequences with the lower case (ac/me3) (me1), (me2), (ph) and (ub) corresponding to modifications of the amino acids by acetylation/trimethylation, monomethylation, dimethylation, phosphorylation and ubiquitination, respectively. (A) ETD spectrum of the ion (M+3H)3+ at m/z 845.5 corresponding to the peptide with the amino acid positions 1–23 in H2AFZ. The peptide is modified by methylation, acetylation/trimethylation, and two phosphorylations. The mass difference of 167 between the fragment ions z13 and z14 corresponds to the phosphorylated serine at the position 10. (B) ETD spectrum of the peptide corresponding to the amino acid positions 2–20; the ion (M+3H)3+ at m/z 670.4. The peptide is modified by two monomethylations, two acetylations/trimethylations and a phosphorylation. The mass difference of 170 between the fragment ions z6 and z7 corresponds to the acetylated/trimethylated lysine at the position 13. (C) ETD spectrum of the peptide corresponding to the amino acid positions 2–20; the ion (M+3H)3+ at m/z 651.8. The peptide is modified by monomethylation, acetylation/trimethylation and phosphorylation. The mass difference of 170 between the fragment ions c14 and c15 corresponds to the acetylated/trimethylated lysine at the position 15. (D) ETD spectrum of the peptide corresponding to the amino acid positions 2–20; the ion (M+3H)3+ at m/z 701.1. The peptide is modified by two ubiquitinations, monomethylation and acetylation/trimethylation. The mass difference of 242 between the fragment ions z12 and z13 corresponds to the ubiquitinated lysine at the position 7. (E) ETD spectrum of the peptide corresponding to the amino acid positions 2–16; the ion (M+3H)3+ at m/z 547.4. The peptide is modified by three monomethylations, phosphorylation and ubiquitination. The mass difference of 242 between the fragment ions z2 and z3 corresponds to the ubiquitinated lysine and the mass difference of 142 between fragment ions z4 and z5 corresponds to the monomethylated lysine. (F) ETD spectrum of the ion (M+4H)4+ at m/z 630.5 corresponding to the peptide with the amino acid positions 1–23 in H2AFZ. The peptide is modified by ubiquitination and three dimethylations. The mass difference of 242 between the fragment ions c11 and c12 and the difference of 141 between the doubly charged fragment ions z112+ and z122+ correspond to the ubiquitinated lysine.