Figure 3. (from Reference 56) Domain-swapped Zipper-Spine Model for the RNase A Protofibril.
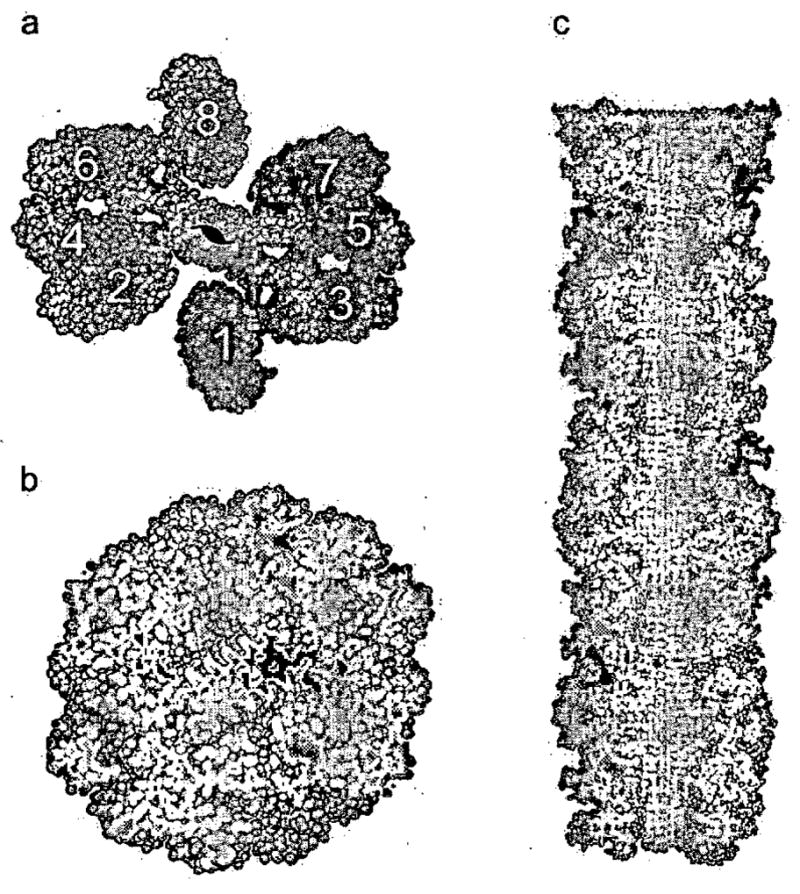
a, The model is a ‘runaway’ domain swap between the RNase A monomers with swaps occurring within one half protofibril but not between half protofibrils. Monomers 1–4 compose half the protofibrillar unit and are coloured as in Fig. 1c to emphasize domain swapping. The C-terminal β-strand of monomer 1 swaps into 2, 2 swaps into 3, and 3 swaps into 4, rising along the axis of the fibril. Q10 segments from these monomers form one antiparallel β-sheet in the spine. Monomers 5–8 form the other β-sheet, related to the monomers 1–4 by a 21 axis along the fibril. Eight RNase A monomers comprise the asymmetric unit of the fibril. A similar model can be built from domain-swapped dimers, and currently available data do not favour one of these models over the other, b, The protofibril cross-section reveals the steric zipper, the interdigitation of Gln sidechains in the spine of the fibril, modelled on the structure of GNNQQNY. c, The protofibril model in longitudinal cross-section. The zipper-spine is seen at the center of the protofibril.
