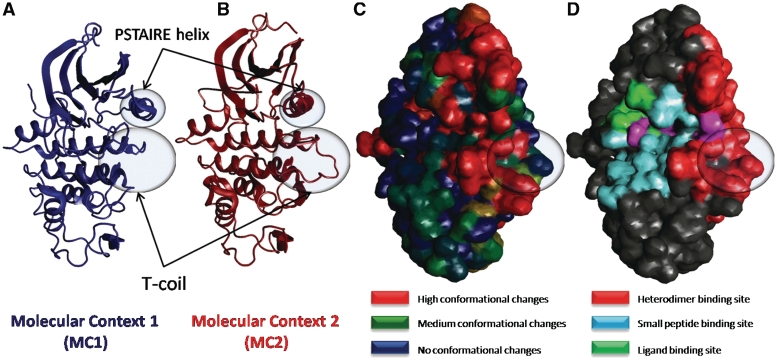
Figure 4.
Change of conformation between molecular contexts: the case of CDK. Molecular Cartography obtained from the structure of the CDK2 (1fin:A). Two molecular contexts are defined: MC1 corresponds to the CDK2 in an environment where it interacts with water and ligand only, whereas MC2 corresponds to an environment where it also interacts with another protein to form a heterodimer. For each of these molecular contexts, the corresponding sets of structures has been automatically detected by M-ORBIS and an averaged backbone is computed and shown in (A and B). Two main conformational changes are involved between MC1 and MC2: the T-Coil helix moves towards the heterodimer binding site, while the PSTAIRE-helix is pushed in the opposite direction. The amount of conformational change has been mapped onto the protein surface in (C); blue, no change; green, small change; red, important change. The molecular cartography shown in (D) allows to correlate these conformational changes with the location of each binding site type.