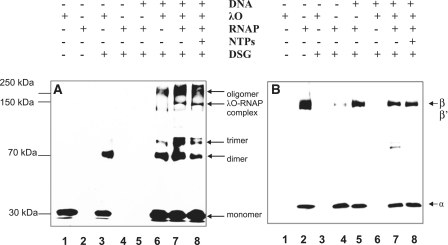
Figure 3.
RNA polymerase interacts with the O-some structure. The λO protein (0.5 µM) was bound to oriλ-containing plasmid (pLamberA; 2.8 nM) and separated from unbound protein fraction by size-exclusion chromatography. Subsequently, reaction was supplemented with RNA polymerase (22 nM) and subjected to DSG cross-linking in the presence (at a final concentration of 0.05 mM) or absence of nucleotides (lanes 6–8). λO and RNA polymerase were subjected to DSG cross-linking without the presence of DNA (lanes 3 and 4, respectively), or RNA polymerase alone was cross-linked in the presence of plasmid DNA (lane 5). Protein complexes were resolved by SDS–PAGE and detected by immunoblotting, performed with polyclonal antibodies specific against λO (A) or a mixture of monoclonal antibodies against α, β and β′ subunits of RNAP (B). The presence or absence of each reaction component is indicated. λO-RNA polymerase complex was depicted by an arrow. Position of monomeric and oligomeric forms of the λO protein was marked.