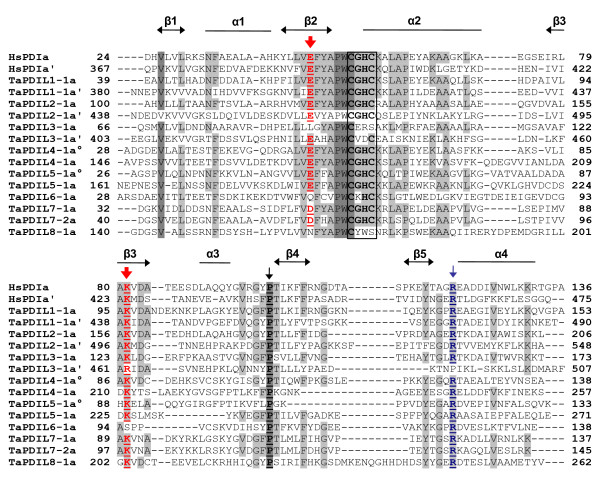
Figure 3.
Multiple sequence alignment of the a-type domains of wheat PDI-like proteins and human typical PDI. The alignment was constructed using ClustalX version 1.83 (Thompson et al. 1997) with the following parameters: gap penalty 10, gap extension penalty 0.5, BLOSUM protein weight matrix. The alignment was also manually adjusted, taking the secondary structure predictions of wheat PDI-like proteins and the known structure of the a domain of the human PDI into account. Residues highlighted in grey and light grey showed 100% and > 50% identity conservation, respectively. The elements of the secondary structure are specified by open bars (α helices) and arrowheads (β strands). Red arrows indicate the two buried charged residues in the vicinity of the active site, a blue arrow indicates the conserved arginine (R) located in the loop between β5 and α4 of the catalitic domains and a black arrow the cis prolines (P) near each active site. Active-site residues within the a type domains are boxed.