Abstract
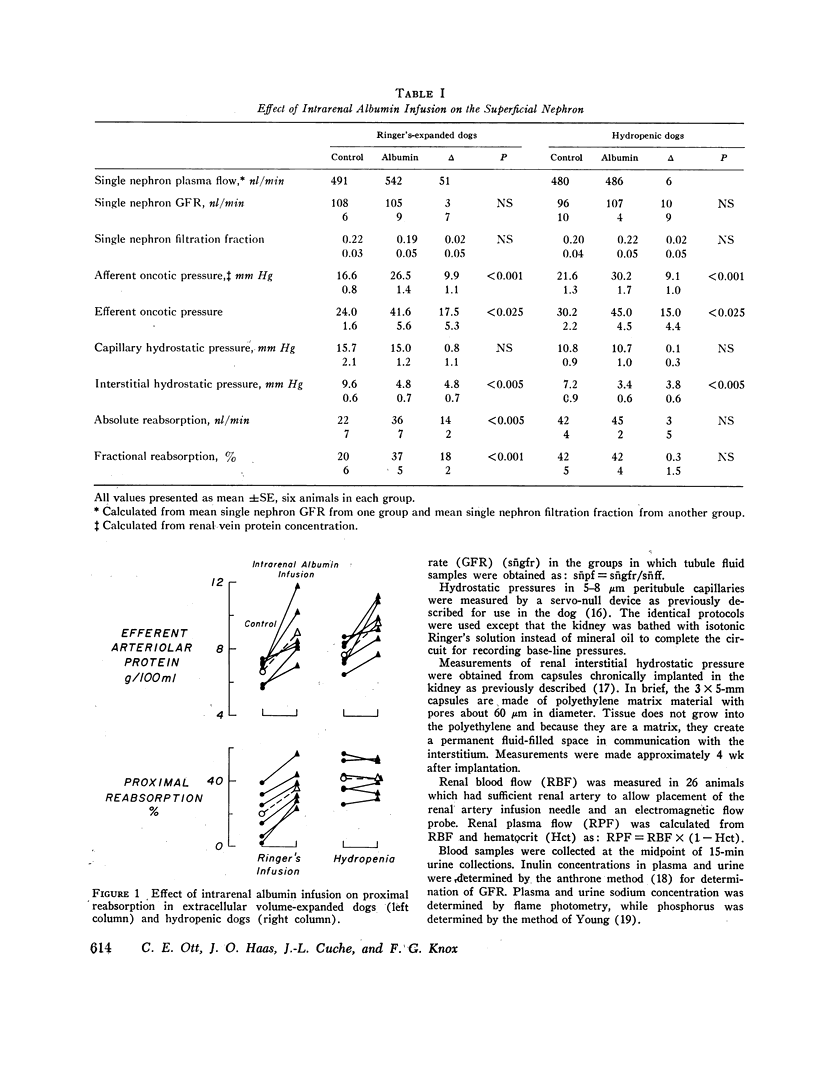
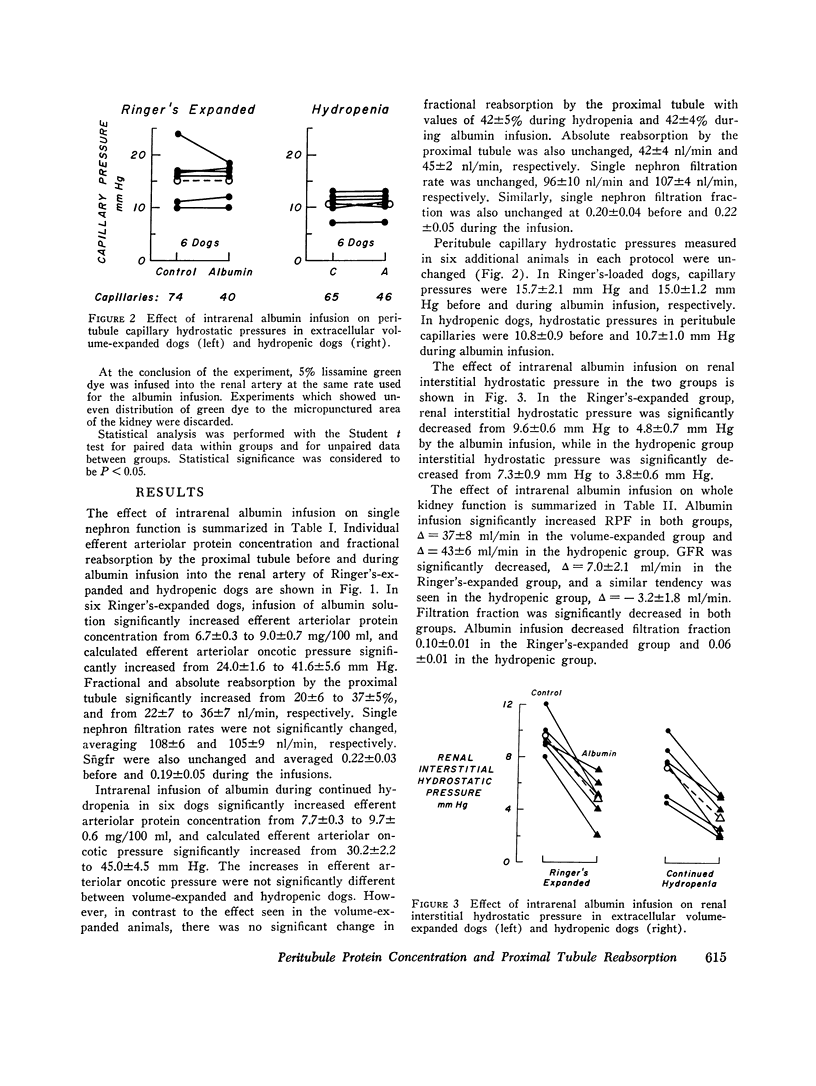
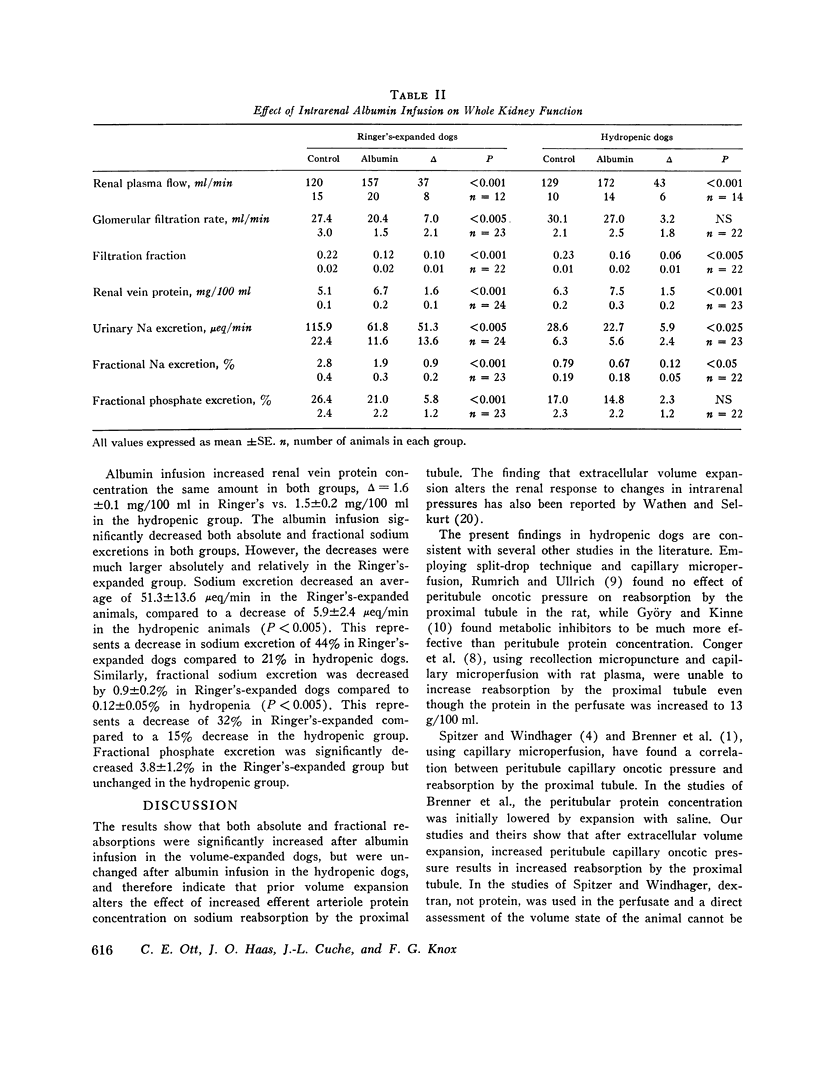
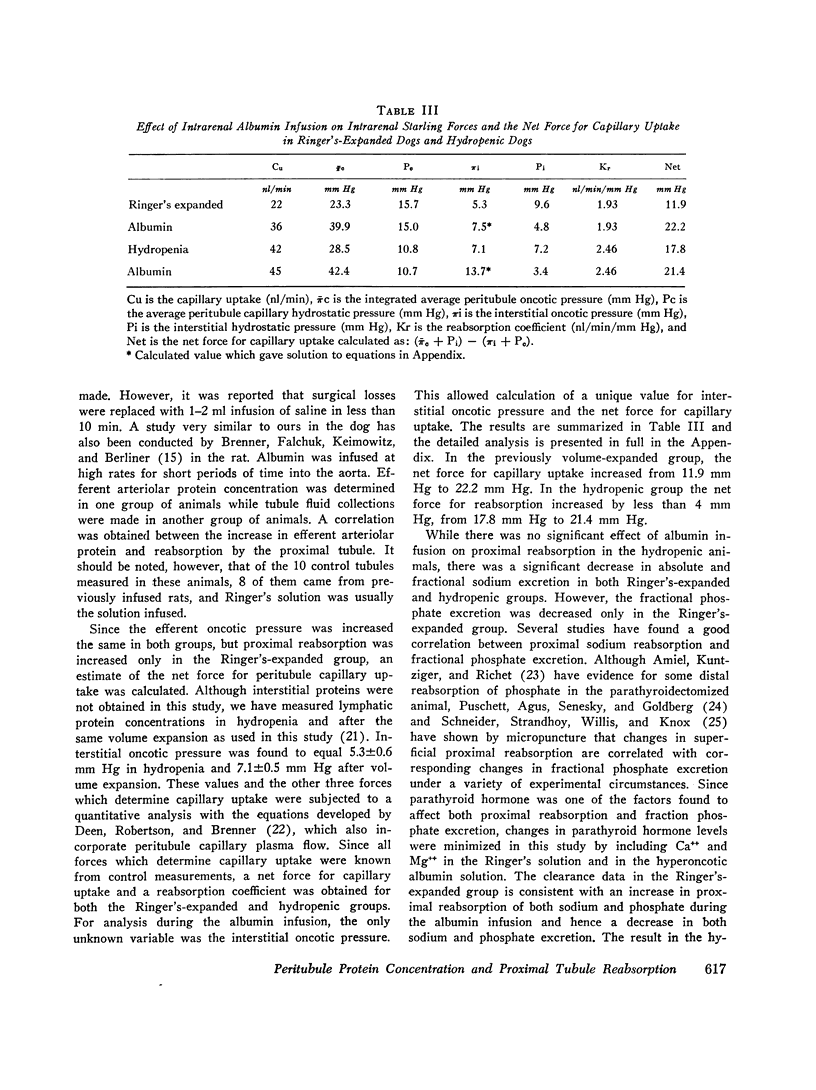
The effect of increased peritubule capillary oncotic pressure on sodium reabsorption by the proximal tubule of the dog was investistigated after extracellular volume expansion (ECVE) with Ringer's solution or during continued hydropenia. Control measurements were made after ECVE or during hydropenia and again during renal arterial infusion with hyperoncotic albumin solution. Absolute reabsorption by the proximal tubule was calculated from fractional reabsorption and single nephron filtration rates as determined by micropuncture. Direct measurements of efferent arteriole protein were used to determine efferent arteriolar oncotic pressure. Albumin infused into the renal artery after ECVE significantly increased efferent oncotic pressure by 17.6 plus or minus 5.3 mm Hg. Fractional and absolute reabsorption by the proximal tubule increased from 20 plus or minus 6 to 37 plus or minus 5% and from 22 plus or minus 6 to 36 plus or minus 7 nl/min, respectively. During hydropenia, the albumin infusion significantly increased efferent oncotic pressure by 15.0 plus or minus 4.4 mm Hg. However, in contrast to the effect seen during ECVE, neither fractional nor absolute reabsorption was changed, delta equals 0.3 plus or minus 1.5% and 3 plus or minus 5 nl/min, respectively. Single nephron filtration rates were not significantly different between the groups and were unchanged by the albumin infusion. Peritubule capillary hydrostatic pressures, measured with a null-servo device, were not changed by the albumin infusion in either group. Renal interstitial hydrostatic pressure, measured from chronically implanted polyethylene capsules, was decreased significantly from 7.2 plus or minus 0.9 to 3.4 plus or minus 0.6 mm Hg in the hydropenic group and from 0.6 plus or minus 0.6 to 4.8 plus or minus 0.7 mm Hg in the Ringer's expanded group. In the hydropenic group, the increase in efferent oncotic pressure was nearly compensated for by changes in interstitial forces so that the calculated net force for capillary uptake was almost unchanged, 17.8 mm Hg before vs. 21.4 mm Hg during the albumin infusion. The increased efferent oncotic pressure in the Ringer's expanded group was not compensated, so that the calculated net force for uptake was increased, 11.9 mm Hg before to 22.2 mm Hg during the albumin infusion. Thus, while the increase in efferent oncotic pressure during albumin infusion was not significantly different between the groups, absolute and fractional reabsorptions were increased only in the animals in which the extracellular volume was expanded. The results suggest that ECVE alters the effect of increased peritubule oncotic pressure on sodium reabsorption by the proximal tubule.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amiel C., Kuntziger H., Richet G. Micropuncture study of handling of phosphate by proximal and distal nephron in normal and parathyroidectomized rat. Evidence for distal reabsorption. Pflugers Arch. 1970;317(2):93–109. doi: 10.1007/BF00592495. [DOI] [PubMed] [Google Scholar]
- Bentzel C. J. Expanding drop analysis of Na and H2O flux across Necturus proximal tubule. Am J Physiol. 1974 Jan;226(1):118–126. doi: 10.1152/ajplegacy.1974.226.1.118. [DOI] [PubMed] [Google Scholar]
- Boulpaep E. L. Permeability changes of the proximal tubule of Necturus during saline loading. Am J Physiol. 1972 Mar;222(3):517–531. doi: 10.1152/ajplegacy.1972.222.3.517. [DOI] [PubMed] [Google Scholar]
- Brand P. H., Cohen J. J. Effect of renal arterial infusion rate on distribution of radioisotopes in kidney. J Appl Physiol. 1972 Nov;33(5):627–634. doi: 10.1152/jappl.1972.33.5.627. [DOI] [PubMed] [Google Scholar]
- Brenner B. M., Falchuk K. H., Keimowitz R. I., Berliner R. W. The relationship between peritubular capillary protein concentration and fluid reabsorption by the renal proximal tubule. J Clin Invest. 1969 Aug;48(8):1519–1531. doi: 10.1172/JCI106118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner B. M., Troy J. L., Daugharty T. M. On the mechanism of inhibition in fluid reabsorption by the renal proximal tubule of the volume-expanded rat. J Clin Invest. 1971 Aug;50(8):1596–1602. doi: 10.1172/JCI106647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner B. M., Troy J. L. Postglomerular vascular protein concentration: evidence for a causal role in governing fluid reabsorption and glomerulotublar balance by the renal proximal tubule. J Clin Invest. 1971 Feb;50(2):336–349. doi: 10.1172/JCI106501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugharty T. M., Belleau L. J., Martino J. A., Earley L. E. Interrelationship of physical factors affecting sodium reabsorption in the dog. Am J Physiol. 1968 Dec;215(6):1442–1447. doi: 10.1152/ajplegacy.1968.215.6.1442. [DOI] [PubMed] [Google Scholar]
- Daugharty T. M., Ueki I. F., Mercer P. F., Brenner B. M. Dynamics of glomerular ultrafiltration in the rat. V. Response to ischemic injury. J Clin Invest. 1974 Jan;53(1):105–116. doi: 10.1172/JCI107527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deen W. M., Robertson C. R., Brenner B. M. A model of peritubular capillary control of isotonic fluid reabsorption by the renal proximal tubule. Biophys J. 1973 Apr;13(4):340–358. doi: 10.1016/S0006-3495(73)85989-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earley L. E., Humphreys M. H., Bartoli E. Capillary circulation as a regulator of sodium reabsorption and excretion. Circ Res. 1972 Sep;31(9 Suppl):1–18. [PubMed] [Google Scholar]
- FUHR J., KACZMARCZYK J., KRUTTGEN C. D. Eine einfache colorimetrische Methode zur Inulinbestimmung für Nieren-Clearance-Untersuchungen bei Stoffwechselgesunden und Diabetikern. Klin Wochenschr. 1955 Aug 1;33(29-30):729–730. doi: 10.1007/BF01473295. [DOI] [PubMed] [Google Scholar]
- Falchuk K. H., Brenner B. M., Tadokoro M., Berliner R. W. Oncotic and hydrostatic pressures in peritubular capillaries and fluid reabsorption by proximal tubule. Am J Physiol. 1971 May;220(5):1427–1433. doi: 10.1152/ajplegacy.1971.220.5.1427. [DOI] [PubMed] [Google Scholar]
- Györy A. Z., Kinne R. Energy source for transepithelial sodium transport in rat renal proximal tubules. Pflugers Arch. 1971;327(3):234–260. doi: 10.1007/BF00586861. [DOI] [PubMed] [Google Scholar]
- Imai M., Kokko J. P. Effect of peritubular protein concentration on reabsorption of sodium and water in isolated perfused proxmal tubules. J Clin Invest. 1972 Feb;51(2):314–325. doi: 10.1172/JCI106816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox F. G., Schneider E. G., Willis L. R., Strandhoy J. W., Ott C. E. Effect of volume expansion on sodium excretion in the presence and absence of increased delivery from superficial proximal tubules. J Clin Invest. 1973 Jul;52(7):1642–1646. doi: 10.1172/JCI107344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox F. G., Willis L. R., Strandhoy J. W., Schneider E. G. Hydrostatic pressures in proximal tubules and peritubule capillaries in the dog. Kidney Int. 1972 Jul;2(1):11–16. doi: 10.1038/ki.1972.64. [DOI] [PubMed] [Google Scholar]
- Knox F. G., Willis L. R., Strandhoy J. W., Schneider E. G., Navar L. G., Ott C. E. Role of peritubule Starling forces in proximal reabsorption following albumin infusion. Am J Physiol. 1972 Oct;223(4):741–749. doi: 10.1152/ajplegacy.1972.223.4.741. [DOI] [PubMed] [Google Scholar]
- Puschett J. B., Agus Z. S., Senesky D., Goldberg M. Effects of saline loading and aortic obstruction on proximal phosphate transport. Am J Physiol. 1972 Oct;223(4):851–857. doi: 10.1152/ajplegacy.1972.223.4.851. [DOI] [PubMed] [Google Scholar]
- Schneider E. G., Lynch R. E., Willis L. R., Knox F. G. Single-nephron filtration rate in the dog. Am J Physiol. 1972 Mar;222(3):667–673. doi: 10.1152/ajplegacy.1972.222.3.667. [DOI] [PubMed] [Google Scholar]
- Schneider E. G., Strandhoy J. W., Willis L. R., Knox F. G. Relationship between proximal sodium reabsorption and excretion of calcium, magnesium and phosphate. Kidney Int. 1973 Dec;4(6):369–376. doi: 10.1038/ki.1973.133. [DOI] [PubMed] [Google Scholar]
- Spitzer A., Windhager E. E. Effect of peritubular oncotic pressure changes on proximal tubular fluid reabsorption. Am J Physiol. 1970 Apr;218(4):1188–1193. doi: 10.1152/ajplegacy.1970.218.4.1188. [DOI] [PubMed] [Google Scholar]
- Stein J. H., Congbalay R. C., Karsh D. L., Osgood R. W., Ferris T. F. The effect of bradykinin on proximal tubular sodium reabsorption in the dog: evidence for functional nephron heterogeneity. J Clin Invest. 1972 Jul;51(7):1709–1721. doi: 10.1172/JCI106972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strandhoy J. W., Ott C. E., Schneider E. G., Willis L. R., Beck N. P., Davis B. B., Knox F. G. Effects of prostaglandins E1 and E2 on renal sodium reabsorption and Starling forces. Am J Physiol. 1974 May;226(5):1015–1021. doi: 10.1152/ajplegacy.1974.226.5.1015. [DOI] [PubMed] [Google Scholar]
- Wathen R. L., Selkurt E. E. Intrarenal regulatory factors of salt excretion during renal venous pressure elevation. Am J Physiol. 1969 Jun;216(6):1517–1524. doi: 10.1152/ajplegacy.1969.216.6.1517. [DOI] [PubMed] [Google Scholar]
- Young D. S. Improved method for the automatic determination of serum inorganic phosphate. J Clin Pathol. 1966 Jul;19(4):397–399. doi: 10.1136/jcp.19.4.397. [DOI] [PMC free article] [PubMed] [Google Scholar]


