We read with interest the article by Li-Wan-Po and colleagues on the functional and clinical implications of the recently discovered CYP2C19*17 variant allele [1]. The authors examine the available literature on the influence of the CYP2C19*17 allele on the disposition of a number of clinically used medicines including voriconazole that are metabolized by this enzyme. The authors conclude that while the CYP2C19*17 allele is associated with increased enzymatic activity, the magnitude of these changes is unlikely to be clinically significant with the possible exception of clopidogrel and tamoxifen [1]. While few studies have assessed the impact of CYP2C19*17 on voriconazole, the available evidence suggests its clinical relevance should not yet be discounted.
Voriconazole is indicated as a first line agent in the treatment of invasive pulmonary aspergillosis [2] and is known to be predominantly metabolized by CYP2C19, and to a lesser extent by CYP2C9 and CYP3A4 [3]. Voriconazole displays non-linear pharmacokinetics and high inter-individual variability [4], with CYP2C19 genotype accounting for 49% of the variance observed in apparent oral clearance [5]. Several studies have demonstrated a relationship between voriconazole serum concentrations and clinical efficacy and toxicity [6–8]. Pascual and colleagues prospectively identified that a lack of response to therapy was more common when trough voriconazole concentrations were below 1 mg l−1 (46% treatment failure) than when concentrations exceeded this value (12% treatment failure) [7]. Neurological toxicity due to voriconazole has been associated with concentrations above 5.5 mg l−1[9], highlighting the narrow therapeutic range associated with this antifungal [10].
Wang et al. [11] examined the pharmacokinetics of voriconazole following a single oral dose in CYP2C19*17 heterozygotes (CYP2C19*1/*17) compared with homozygous extensive metabolizers (CYP2C19*1/*1) and poor metabolizers (CYP2C19*2/*2) [11], as discussed in the review by Li-Wan-Po et al.[1]. While the sample size was small, CYP2C19*17 heterozygotes were found to have a significantly lower systemic exposure compared with homozygous extensive metabolizers (mean AUC0–24 3.39 and 6.18 µg h ml−1, respectively) [11]. The 95% confidence intervals for the AUC0–24 of voriconazole in people with these two genotypes do not overlap (Figure 1). Significant differences were also found between people carrying different genotypes in voriconazole half-life and clearance [11]. A later pharmacokinetic study by Weiss et al. also found that subjects carrying the CYP2C19*17 allele had a lower peak and total voriconazole exposure compared with homozygous extensive metabolizers (CYP2C19*1/*1) [5], although the magnitude of the differences were smaller than those found by Wang et al. [11].
Figure 1.
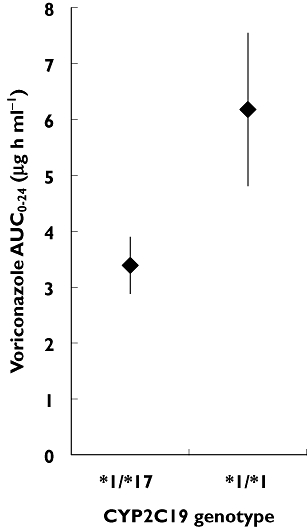
Mean AUC0–24 and 95% confidence intervals for CYP2C19*1/*17 heterozygotes (n = 4) and CYP2C19*1/*1 homozygotes (n = 8) from Wang et al. [11]
Taken together, these studies demonstrate voriconazole exposure is reduced in subjects carrying a single CYP2C19*17 allele, potentially by up to half compared with homozygous extensive metabolizers [11]. These findings suggest subjects carrying a CYP2C19*17 allele may be at greater risk of sub-therapeutic voriconazole concentrations, and subsequently of treatment failure. The routine use of therapeutic drug monitoring for voriconazole [9] would identify low concentrations and allow dose adjustment in such patients. However, the association of low initial trough voriconazole concentrations with increased mortality [8] implies this may not ensure a positive clinical outcome in all cases.
The authors are not aware of any published studies that have investigated voriconazole metabolism in CYP2C19*17 homozygotes (CYP2C19*17/*17). It may be expected that these subjects would display further reduced voriconazole exposure when compared with homozygous extensive metabolizers, as is the case for omeprazole [1]. Furthermore, no studies have yet assessed the impact of the CYP2C19*17 allele on the pharmacokinetics of voriconazole at steady state. Considering the clinical consequences of low voriconazole concentrations, we recommend awaiting the results of larger, multiple dose studies including CYP2C19*17 homozygotes before discounting the clinical importance of the CYP2C19*17 allele for voriconazole therapy.
Competing interests
There are no competing interests to declare.
REFERENCES
- 1.Li-Wan-Po A, Girard T, Farndon P, Cooley C, Lithgow J. Pharmacogenetics of CYP2C19: functional and clinical implications of a new variant CYP2C19*17. Br J Clin Pharmacol. 2010;69:222–30. doi: 10.1111/j.1365-2125.2009.03578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walsh TJ, Anaissie EJ, Denning DW, Herbrecht R, Kontoyiannis DP, Marr KA, Morrison VA, Segal BH, Steinbach WJ, Stevens DA, van Burik J-A, Wingard JR, Patterson TF, America IDSo Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis. 2008;46:327–60. doi: 10.1086/525258. [DOI] [PubMed] [Google Scholar]
- 3.Theuretzbacher U, Ihle F, Derendorf H. Pharmacokinetic/pharmacodynamic profile of voriconazole. Clin Pharmacokinet. 2006;45:649–63. doi: 10.2165/00003088-200645070-00002. [DOI] [PubMed] [Google Scholar]
- 4.Andes D, Pascual A, Marchetti O. Antifungal therapeutic drug monitoring: established and emerging indications. Antimicrob Agents Chemother. 2009;53:24–34. doi: 10.1128/AAC.00705-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weiss J, Ten Hoevel MM, Burhenne J, Walter-Sack I, Hoffmann MM, Rengelshausen J, Haefeli WE, Mikus G. CYP2C19 genotype is a major factor contributing to the highly variable pharmacokinetics of voriconazole. J Clin Pharmacol. 2009;49:196–204. doi: 10.1177/0091270008327537. [DOI] [PubMed] [Google Scholar]
- 6.Denning DW, Ribaud P, Milpied N, Caillot D, Herbrecht R, Thiel E, Haas A, Ruhnke M, Lode H. Efficacy and safety of voriconazole in the treatment of acute invasive aspergillosis. Clin Infect Dis. 2002;34:563–71. doi: 10.1086/324620. [DOI] [PubMed] [Google Scholar]
- 7.Pascual A, Calandra T, Bolay S, Buclin T, Bille J, Marchetti O. Voriconazole therapeutic drug monitoring in patients with invasive mycoses improves efficacy and safety outcomes. Clin Infect Dis. 2008;46:201–11. doi: 10.1086/524669. [DOI] [PubMed] [Google Scholar]
- 8.Miyakis S, van Hal SJ, Ray J, Marriott D. Voriconazole concentrations and outcome of invasive fungal infections. Clin Microbiol Infect. 2010;16:927–33. doi: 10.1111/j.1469-0691.2009.02990.x. [DOI] [PubMed] [Google Scholar]
- 9.Pasqualotto AC, Xavier MO, Andreolla HF, Linden R. Voriconazole therapeutic drug monitoring: focus on safety. Expert Opin Drug Saf. 2010;9:125–37. doi: 10.1517/14740330903485637. [DOI] [PubMed] [Google Scholar]
- 10.Brüggemann RJM, Donnelly JP, Aarnoutse RE, Warris A, Blijlevens NMA, Mouton JW, Verweij PE, Burger DM. Therapeutic drug monitoring of voriconazole. Ther Drug Monit. 2008;30:403–11. doi: 10.1097/FTD.0b013e31817b1a95. [DOI] [PubMed] [Google Scholar]
- 11.Wang G, Lei H-P, Li Z, Tan Z-R, Guo D, Fan L, Chen Y, Hu D-L, Wang D, Zhou H-H. The CYP2C19 ultra-rapid metabolizer genotype influences the pharmacokinetics of voriconazole in healthy male volunteers. Eur J Clin Pharmacol. 2009;65:281–5. doi: 10.1007/s00228-008-0574-7. [DOI] [PubMed] [Google Scholar]


