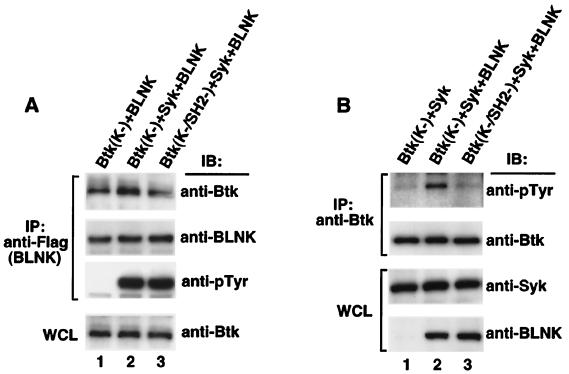
Figure 2.
Syk-dependent Btk phosphorylation requires the interaction of BLNK and Btk-SH2 domain. cDNAs of Btk [Btk(K−) or Btk(K−/SH2−)] were cotransfected into 293T cells with the indicated combinations of Syk and/or BLNK. (A) Assessments of the coprecipitation of Btk and BLNK were performed by tagging BLNK with Flag sequence as described in our previous report (12). Flag-tagged BLNK was immunoprecipitated from cell lysates with anti-Flag mAb M2. Immune complexes were immunoblotted with anti-Btk mAb 43-3B for detecting the coprecipitation of Btk (Top), followed by reprobing with anti-BLNK Ab (Second Panel). The Syk-dependent tyrosinephosphorylation of BLNK was detected by immunoblotting with anti-pTyr mAb 4G10 (Third Panel). Equal expression levels of Btk, Syk, and BLNK in each experiment were confirmed by immunoblotting the whole cell lysates with anti-Btk mAb 43-3B (Bottom), anti-Syk Ab and anti-BLNK Ab (not shown). (B) Btk was immunoprecipitated from cell lysates with anti-Btk mAb 48-2H, and the tyrosinephosphorylation was evaluated by anti-pTyr mAb 4G10 (Top). The filter was reprobed with anti-Btk mAb 43-3B to confirm the equal amount of precipitated Btk (Second Panel). The whole cell lysates were immunoblotted with the anti-Syk Ab (Third Panel) or the anti-BLNK Ab (Bottom).