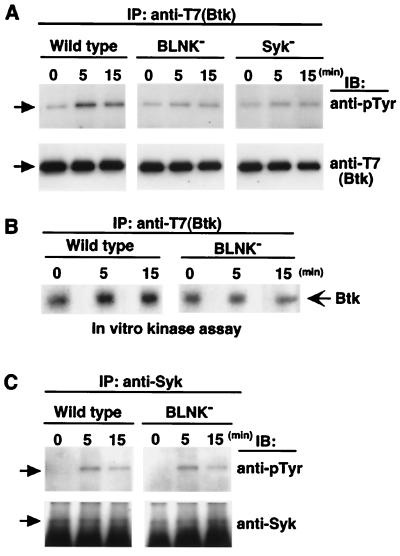
Figure 4.
BCR-induced tyrosinephosphorylation and activation of Btk are reduced in BLNK-deficient DT40 cells. Wild-type or mutant (BLNK-deficient and Syk-deficient) DT40 cells expressing T7-Btk were stimulated with anti-chicken IgM mAb M4 (4 μg/ml) for the indicated periods. Lysates were immunoprecipitated with the anti-T7 mAb (A) or the anti-Syk Ab (C), and then immunoblotted with anti-pTyr mAb 4G10 (A and C, Top), followed by reprobing with the anti-T7 mAb (A, Bottom) or the anti-Syk Ab (C, Bottom). (B) After stimulation of wild-type and BLNK-deficient DT40 cells with mAb M4, T7-Btk was immunoprecipitated with the anti-T7 mAb, and an in vitro kinase assay was carried out as described in Materials and Methods. The equality of Btk protein in immunoprecipitates was confirmed by anti-Btk immunoblotting (data not shown).