Abstract
Waveguide numerical aperture restrictions and light-blocking elements are used to create a microfluidic cytometer with both illumination and two-parameter light scatter collection systems integrated on-chip. Good forward scatter coefficients of variation (9.7-18.3%) are achieved for polystyrene beads under a reasonably high flow rate (28 cm/s) using a greatly simplified optical system.
OCIS codes: (120.5820) Scattering measurements, (130.3120) Integrated optics devices, (170.0170) Biomedical optics and biotechnology, (230.3890) Micro-optical devices
1. Introduction
The flow cytometer is a well-known diagnostic tool incorporating both optical and fluidic systems, making it an excellent and widely applicable test bed for lab-on-a-chip and optofluidic technologies. The cytometer consists of a fluid flowing single analytes one by one through a laser beam. Scattered light is collected at different angles (generally near 0° and at 90° from the illumination direction) and induced fluorescence is also detected. These parameters allow for the identification of various subpopulations of analytes, yielding statistics about relative population size, etc. that can relate to many aspects of health [1]. A lab-on-a-chip cytometer could revolutionize the field of flow cytometry, increasing availability and decreasing system cost. Practically speaking, the device must operate at a reasonable throughput (capable of sample flow of at ~106 samples/mL and >10 μL/min) and deliver quality data with enough parameters (2 + ) to perform useful analysis. Data quality can be demonstrated, for example, by showing coefficients of variation (CVs) similar to benchtop CVs or manufacturer-defined CVs (e.g. for polystyrene beads, often <3-5%).
Relatively effective fluorescence detection has been demonstrated numerous times using microscope optics or similar setups [2–4], however little attention has been paid to the important gating parameters of light scattering, and even less attention has been paid to integrating such optical systems. A chip with mass-fabricated, pre-aligned optical and fluidic systems could eliminate many operational issues and maintenance requirements by allowing simple replacement upon clogging and circumventing the issue of optical misalignment and drift. Progress has been made detecting side scatter off-chip [2,5]. Several approaches to simplified and integrated optical systems have shown promise, but CVs for both scattering parameters remain high (25-30% for polystyrene beads) and geometry constraints often force collection lines to nonstandard locations, causing problems with data analysis and comparison to commercial devices [6,7]. The high CVs suggest a need for (1) improved sample localization via improved flow control and (2) improved signal-to-noise ratios (SNR) in light collection. In particular, the use of simple fiber optics or waveguides is problematic for light collection as these components accept light over their entire numerical aperture, effectively allowing a substantial degree of noise to contaminate the signal. Integrated lenses offer one approach to this problem [7–9], but non-imaging approaches may offer more elegant solutions, given the great design flexibility permitted by mold-replicated devices.
In this work we demonstrate localized (angularly-resolved/background-reduced) light scatter collection via an exclusion-based (lensless) approach to the optical design, a means of improving the system SNR made possible only by the microfabricated nature of integrated microfluidic chips. On the chip, optical signals pass through a minimal number of surfaces, each with a low refractive index contrast (Δn<0.02), while much of the excess collection typically involved in a waveguide-based approaches is mitigated by a combination of light blocking elements and customized waveguide structures that either reflect or redirect light originating from locations other than the sample of interest. The angles detected are those traditionally collected in commercial devices: forward scatter (‘FSC’, here ~3-12°) and side scatter (‘SSC’, here ~82-98°). The detectors employed are the typical photodiode and photomultiplier tube (PMT), respectively. In addition to improvements in the optical system, a previously-demonstrated three-dimensional flow focusing system was implemented to improve sample confinement [3]. Results from a mixed sample of 5 μm, 10 μm, and 15 μm beads run on both our microfluidic device and a commercial device allow us to demonstrate our device's ability to distinguish bead subpopulations, benchmark performance with bead CVs, and draw comparisons to existing technologies. The results demonstrate a strong throughput (sample flow at 10 μL/min; total flow at 100 μL/min) and the achievement of CVs that compare very favorably with published values, making strong strides towards the goal of competing with simple commercial devices but using drastically simplified optical systems.
2. Device design
The device is a PDMS (polydimethylsiloxane) polymer-molded chip integrating optical waveguides, microfluidic channels, and baffles (elements to block stray light). The chip employs a number of previously-demonstrated features, such as two-dimensional flow focusing [4–6,10], integrated waveguides [2,7,9,11], and waveguide-fiber coupling sleeves [2,10]. Our current chip does not include complicated optical systems or components, which can be difficult to fabricate in a chip environment, but possess a unique architecture, discussed below.
Three-dimensional flow focusing is known to be of great importance in reducing population variation in flow cytometry, as changes in sample positioning will equate to changes in travel velocity. This effect is increasingly important as sample size decreases. In an attempt to address this issue, we included the typical lateral flow focusing, and also mimicked the chevron-shaped patterns above and below the channel demonstrated by the Ligler lab in order to achieve integrated focusing in the vertical dimension (see Fig. 1 ) [2]. Flowing dye through the sample channel and viewing the device through the side confirms improvements in vertical flow confinement of the sample fluid. All branches of the fluidic channel are 50 μm wide and roughly 60 μm tall, all combining into a single 100 μm wide channel. The four chevron structures are approximately 25 μm in depth, 50 μm thick, with an edge-to-edge spacing of 70 μm. To allow room for FSC and orthogonal scatter (SSC) on the same chip, the fluidic channel was slightly tilted with respect to the illumination line. The integrated optical components include an input waveguide for the interrogation laser beam, custom-shaped output waveguides for FSC and SSC detection, blackened baffle areas for stray light suppression, and a beam dump structure to remove light from outside of the intended FSC and SSC detection areas.
Fig. 1.
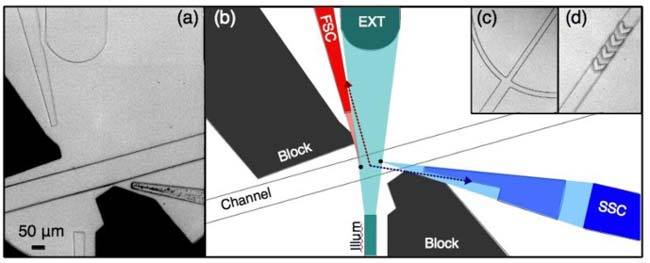
(a) Microscope image of the microfluidic device. (b) Scale schematic of device showing light scatter (‘FSC’ and ‘SSC’) collected by waveguides from interrogation centers (two black circles in channel). Note that light originating from between these centers is incident on an angled facet (see arrows), resulting in large reflection or refraction losses. After traversing the fluidic channel, the illumination beam is guided off the chip by the beam dump, which by nature will collect the ‘extinction’ signal (EXT), a dip in intensity as samples pass. c) Typical fork-style lateral hydrodynamic focusing and (d) chevron-based vertical focusing are used to confine sample flow.
Figure 1(a) shows a microscope image of the interrogation region of the device, while Fig. 1(b) shows a scale schematic. All features (except chevrons) are approximately 60 μm tall. A 50 μm wide waveguide brings light onto the chip to interrogate the sample. A larger, slightly lensed ‘beam dump’ waveguide collects much of this light after it has traversed the microfluidic channel (i.e. extinction). Two custom-shaped waveguides are used to collect FSC and SSC. Baffles, or stray light blocks, were filled with a black PDMS that is cured into a solid. At the device edges (not shown), all waveguides taper down to 50 μm in diameter to interface with optical fibers via simple fiber sleeves.
In this device, light diverges from the illumination waveguide with an angular range spanning nearly 14° ( ± 7° from optical axis), as is typical for on-chip illumination. In this work, however, two separate locations are used for interrogation, indicated by circles in the microfluidic channel in Fig. 1(b) and referred to as the FSC (leftmost) and SSC (rightmost) interrogation points, respectively. By utilizing only the extreme edges of the exiting light, a more limited range of illumination angles (approximately 3°) in ensured relative to the typical approach of using the beam. It should be noted that the illumination waveguide is multimode (λ = 488 nm, waveguide diameter = 50 μm). Commercial devices use free-space optics along with highly single-mode lasers to ensure a stable beam profile, as disruption to mode purity of even 1-2% can significantly affect sample CVs [12]. In our design, we use a large waveguide that support more than a thousand modes. Due to the large number of modes, the noise produced by mode hopping and multi-mode interference (MMI) is suppressed, resulting in a more stable radiation pattern. This sort of extremely multimode excitation should be permissible as the resulting intensity variations will be minimal and occur over much smaller length scales as compared with low-order modal variation. Polarization-dependent scatter measurements (an occasionally useful but infrequently utilized technique) will not be possible in this device.
The second performance-enhancing feature, the use of angularly-based light exclusion in the two scatter collection lines, is affected by a combination of customized light guiding elements (waveguides) as well as the use of light blocking elements (baffles). At the FSC interrogation location, the collection waveguide collects and guides light scattered at ~3-12° from the interrogation direction. The SSC line collects light scattered at ~82°-98° from the interrogation direction. Each collection line begins with a small flat facet that expands with the desired collection cone, tapering into a rectangular waveguide. For the SSC waveguide, this portion was disconnected from the waveguide to allow filling with a higher-index material. It was also effectively split into two portions for similar geometry concerns. For signal collection, the intended collection cone is readily coupled into what is effectively seen as a flat-facet waveguide (the small end of the tapered portions). On the other hand, much of the light originating from outside of the interrogation regions is incident on the angled facets of the tapered waveguide. At such shallow incidence angles, much of this ‘noise’ will be reflected. The light that does refract through the angled facet is generally sufficiently shifted in propagation direction (Snell’s Law) to prevent total internal reflection (TIR) once the light reaches the parallel portion of the waveguide. Thus the locations of the waveguides were chosen to include the signal from the cells, but the main design feature, the unusual geometry of the waveguides, is focused primarily on excluding this excess light (hence the idea of an exclusion-focused design). In order for such a system to perform well, the ends of the tapered waveguides must be quite small (here ~20 μm) and located very close to the sample; the further these facets are located from the sample the larger they must be to collect the desired cone of light, and thus the more excess light they will collect (approaching simple flat-facet waveguide performance). In this way, the unique approach employed in this work is enabled only by the fact that such a chip can be created via microfabrication.
3. Device fabrication
Devices are fabricated using soft lithography methods [13] in a similar fashion to those employed to create devices in our previous work [10]. A silicon mold master is used to make polydimethylsiloxane (PDMS; Sylgard 184, Dow Corning) replicas. For the lithography, a printed transparency (CAD/Art Services, Bandon, Oregon, USA) at 20,000 dpi is used as a photomask. Using standard lithography practices, the pattern is transferred to a layer of 70 μm thick SU-8 50 photoresist (MicroChem Corp.). The body of the device is made from Gelest PDMS (nd~1.41; OE41, Gelest Inc). After curing, two replicas (from the mold and from a flat) are bonded with UV/Ozone cleaner (UVO-Cleaner 42, Jelight Inc.). The baffles are filled with black-colored PDMS (Sylgard 170, Dow Corning). Waveguides are filled with another transparent Gelest PDMS, this time of a higher refractive index (nd~1.42, OE42, Gelest) using vacuum and subsequent pressure and capillary action forces. Cut, cleaved, and cleaned fibers are inserted into the fiber sleeve portion of the device to aid in coupling light into the device. Holes are punched to access the fluidic channels directly with Tygon tubing.
4. Experimental conditions and setup
Spherotech size calibration beads (PPS-6K, Spherotech, Inc.; this work uses only the 5 μm, 10 μm, and 15 μm beads) were suspended in 0.22 μm filtered DI water. The solution is pumped into the device via a syringe at a rate of 10 μL/min, while the sheath flow channels are fed by a single syringe of 0.22 μm filtered DI water being pumped at 90 μL/min. The expected equivalent flow velocity in the 100 μm x 60 μm channel (neglecting the parabolic flow profile) is approximately 28 cm/sec. The commercial device was operated at its ‘slow’ flow setting, i.e. sample flow at 14 μL/min and a core size of 14 μm.
For the microfluidic device, the interrogation region is illuminated with a 488 nm laser (Cyan 40 mW, Newport Corporation) with a multimode fiber-coupled output (OzOptics, Canada). The output fiber interfaces with bare multimode fiber (GIF50, Thor Labs) inserted into the fiber sleeve of the illumination line via a bare fiber adapter (FC connected, Newport). After these connections, the total power entering the device is substantially less than the 40 mW the laser emits. Fibers carrying collected light (FSC and SSC lines) interface with detectors via a fiber adapter (SM1FC, Thor Labs). Signal from the FSC line is detected via a battery-biased silicon detector (DET36A, Thor Labs) and amplified (PDA 6424, ILX Lightwave) before acquisition. Signal from the SSC line is detected by a PMT (H10425, Hamamatsu) and amplified by an external preamplifier with a 20 kHz bandwidth and 105 gain (C7319, Hamamatsu). Both systems (photodiode and PMT) connected via BNC to a connector block (NI BNC 2110, National Instruments), allowing signal capture by a data acquisition card (NI PCI-6251, National Instruments) using off-the-shelf software (Signal Express, National Instruments). Acquisition occurs at 50 kHz over 3 channels. Data from the beam dump is not used for population discrimination this work, however this line does show the expected extinction dips associated with bead passage. In the experiments using the commercially-available benchtop cytometer, the standard 488 nm illumination source is used.
5. Results
Logged signal data was converted to ASCII and imported into Matlab. Custom code performed DC baseline removal through mean subtraction and mild high-pass filter. A simple peak-finding algorithm based on a user-defined threshold and a pulse-width minimum generates an 'event' list. A sample of this data is shown in Fig. 2 . A total of 12,985 events (see Table 1 ) were recorded in a nearly 5 minute data run, for an average throughput of 43 beads/sec. The event list was converted to standard .FCS (flow cytometry standard) format using the A2FCS routine offered as part of the MFI (Mean Fluorescence Intensity) software [14]. Some extreme outliers that would have been otherwise gated out were removed from the data set to more clearly display the data.
Fig. 2.
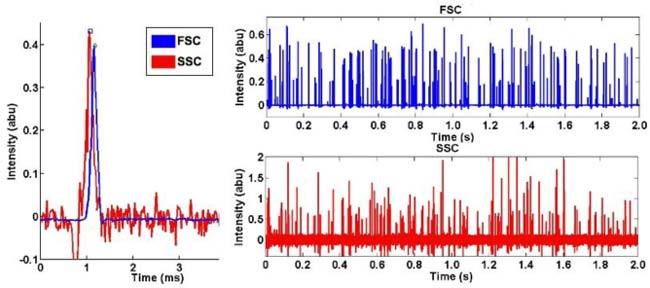
(left) Close-up of a signals from a single bead (scaled to display together), showing a slight time delay between the FSC peak and the SSC peak. (right) Signals recorded over two seconds of data show a large number of beads passing through the device.
Table 1. Number of Beads Analyzed.
| Number of Events: | 5 μm | 10 μm | 15 μm | All Events |
|---|---|---|---|---|
| Our Device: | 1,716 | 5,902 | 4,427 | 12,985 |
| Commercial Device: | 1,493 | 996 | 1,030 | 3,519 |
The converted data from the microfluidic device and data from the commercial device were both analyzed using commercially-available software (FlowJo, Tree Star Inc.). Figure 3(a-b) shows FSC area histograms obtained by utilizing the autogating feature (see Fig. 3 c-d) to gate the distinct bead regions. For the data from the microfluidic device, a simple quadrant gate was also employed to remove the contribution from low-level noise that the thresholding in the commercial device automatically removed. The FSC-SSC scatter plots are shown in Fig. c-d. Table 2 summarizes CVs obtained for each bead population by each device.
Fig. 3.
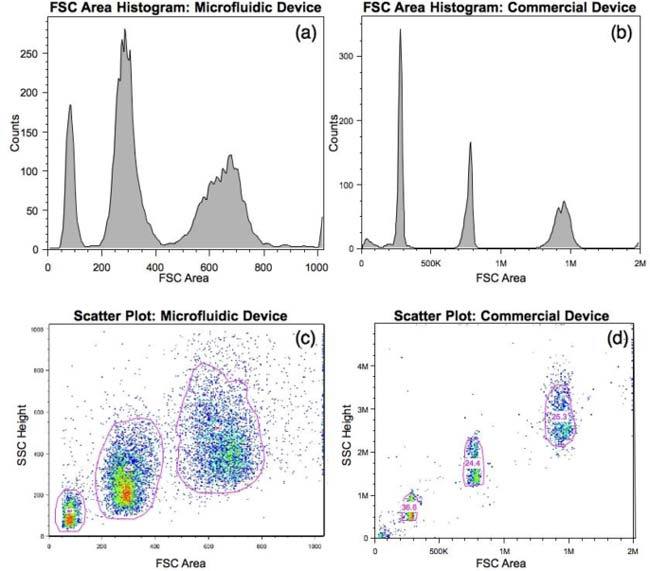
Data plots for a sample of 5, 10, and 15 μm beads from both our device (left column) and the commercial device (right column), including FSC area histograms (a,b) and FSC-SSC scatter plots (c,d). The FSC separation on the microfluidic device is quite clear.
Table 2. Light Scatter Intensity CVs.
| FSC CV (μfluidic) | FSC CV (commercial) | SSC CV (μfluidic) | SSC CV (commercial) | |
|---|---|---|---|---|
| 5 μm | 18.3% | 4.47% | 37.5% | 23.6% |
| 10 μm | 13.2% | 2.86% | 34.2% | 17.5% |
| 15 μm | 9.7% | 3.21% | 27.7% | 11.9% |
6. Discussion
This device demonstrates integrated illumination as well as integrated light scatter collection CVs for two parameters. This is an especially notable achievement due to the need to reduce a typically three-dimensional system (fluid flow, illumination, orthogonal scatter) to an effectively two-dimensional (planar) system when FSC, SSC, and illumination are all desired on-chip. The FSC CVs measured from this device range from 18.3% down to 9.7% (5 μm to 15 μm diameter beads). This compares quite favorably with FSC CVs from previously demonstrated microfluidic devices, which generally range from 25%-30% (1 μm to 9 μm diameter beads) [6,7].
FSC area was utilized in lieu of FSC height. The FSC height CV was broadened slightly, likely due to a dependence on travel velocity from either flow instability (pulsatile flow, bubbles in the lines, etc) or vertically under-confined flow. FSC area seemed less affected by these small variations. No clear effect was seen on SSC height, perhaps due to the already-large CVs for this parameter. In addition, the observed spacing between the FSC and SSC peaks was smaller than expected; the results suggest that the interrogation point separation is closer to 20-25 μm rather than the intended separation of ~70 μm. As a result, the systems likely collected light scatter originating from a larger range of illumination angles than intended. This is likely due to the higher illumination intensity towards the center of the illumination area, shifting the location at which peak intensity collection occurred. This would not affect the SSC results, as commercial devices typically collect light over very large angles. The change would be more likely to modify the character of the FSC, however, no evidence of this problem was observed.
The tapered optic of the FSC line in this device resulted in a 10-fold improvement in SNR over an identical device with only a flat-facet waveguide. Our device is able to resolve 2-3 μm beads (data not shown), however it is not clear that all of the beads in flow were well resolved, so these results were not included. Both collection lines still exhibit some significant background noise levels that ultimately limit amplifier gain and SNR. This is due to a combination of effects, such as scatter due to sidewall roughness in the device, defects in the PDMS waveguides, and imperfections in waveguide-fiber coupling. Reducing this background should enable full discrimination of 2-3 μm beads (and possibly lower). The SSC line in particular could benefit from a reduction in background light levels, allowing for higher PMT gain levels to be utilized to better separate the bead populations. Furthermore, in the case of the SSC line, a larger collection NA would also be expected to benefit the signal strength. Typical commercial devices utilize the same high NA collection optics for SSC as they do for fluorescence, thus the total solid angle collected in our device is significantly lower than that of a commercial device. The resolution of SSC collection in commercial devices, however, is often limited not by total signal collection but rather by noise collection [15]. This suggests that the total solid angle typically collected for SSC is somewhat excessive, and could be reduced if paired with sufficient stray light reduction. Thus while the solid angle collected in our device should be increased, the increase likely does not need to be as drastic as the current disparity between the collection solid angle of this device and commercial devices might suggest.
Under the current flow rates, the device throughput is limited to ~400 samples/sec (to ensure a coincidence loss of 1% or less). This limit could be increased further by increasing flow velocity. Results were observed for this device at total flow rates as high as 200 μL/min (calculated throughput of 800 samples/sec), however this is currently the approximate limit of detection sensitivity for this device. As discussed above, further reducing background noise from rough sidewalls and fabrication imperfections should significantly increase this throughput. In addition, the distance between the two interrogation points can be further reduced if a more collimated illumination source is created (e.g. lower NA illumination waveguide), allowing for further throughput increases.
7. Conclusion
We have demonstrated a high throughput optofluidic flow cytometer capable of measuring dual-parameter light scatter by using an exclusion-based approach to integrated light collection. The device measures forward (~3-12°) and orthogonal (~82-98°) scatter, delivering results that are qualitatively similar to those of a benchtop device, albeit with quantitatively higher intensity variations. The CVs, already some of the best achieved in the field, can be further reduced by improving three-dimensional flow focusing, by lowering background light levels to enable use of the full amplification range of the detectors, by increasing total collection solid angles, and/or by creating smoother sidewalls [2,16]. The architecture and fabrication methods utilized in this work are readily integrated with previously published work from the same group on micro-optics [10] and low-voltage, high-throughput single cell sorters on a microfluidic platform [17]. With future improvements, this device could be capable of performing light scatter based assays, such as relative white blood cell counts. If combined with fluorescence detection, and possibly sorting architectures, a fully-functional, highly-integrated cytometer could be created.
Acknowledgments
This publication was made possible support from the National Institutes of Health. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
References and links
- 1.H. M. Shapiro, Practical Flow Cytometry (Wiley, 2004). [Google Scholar]
- 2.Kummrow A., Theisen J., Frankowski M., Tuchscheerer A., Yildirim H., Brattke K., Schmidt M., Neukammer J., “Microfluidic structures for flow cytometric analysis of hydrodynamically focussed blood cells fabricated by ultraprecision micromachining,” Lab Chip 9(7), 972–981 (2009). 10.1039/b808336c [DOI] [PubMed] [Google Scholar]
- 3.Golden J. P., Kim J. S., Erickson J. S., Hilliard L. R., Howell P. B., Anderson G. P., Nasir M., Ligler F. S., “Multi-wavelength microflow cytometer using groove-generated sheath flow,” Lab Chip 9(13), 1942–1950 (2009). 10.1039/b822442k [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolff A., Perch-Nielsen I. R., Larsen U. D., Friis P., Goranovic G., Poulsen C. R., Kutter J. P., Telleman P., “Integrating advanced functionality in a microfabricated high-throughput fluorescent-activated cell sorter,” Lab Chip 3(1), 22–27 (2003). 10.1039/b209333b [DOI] [PubMed] [Google Scholar]
- 5.Schrum D. P., Culbertson C., Jacobson S., Ramsey J., “Microchip flow cytometry using electrokinetic focusing,” Anal. Chem. 71(19), 4173–4177 (1999). 10.1021/ac990372u [DOI] [PubMed] [Google Scholar]
- 6.Pamme N., Koyama R., Manz A., “Counting and sizing of particles and particle agglomerates in a microfluidic device using laser light scattering: application to a particle-enhanced immunoassay,” Lab Chip 3(3), 187–192 (2003). 10.1039/b300876b [DOI] [PubMed] [Google Scholar]
- 7.Wang Z., El-Ali J., Engelund M., Gotsaed T., Perch-Nielsen I. R., Mogensen K. B., Snakenborg D., Kutter J. P., Wolff A., “Measurements of scattered light on a microchip flow cytometer with integrated polymer based optical elements,” Lab Chip 4(4), 372–377 (2004). 10.1039/b400663a [DOI] [PubMed] [Google Scholar]
- 8.Seo J., Lee L. P., “Disposable integrated microfluidics with self-aligned planar microlenses,” Sens. Actuators B Chem. 99(2-3), 615–622 (2004). 10.1016/j.snb.2003.11.014 [DOI] [Google Scholar]
- 9.Godin J., Lien V., Lo Y., “Demonstration of two-dimensional fluidic lens for integration into microfluidic flow cytometers,” Appl. Phys. Lett. 89(6), 061106 (2006). 10.1063/1.2266887 [DOI] [Google Scholar]
- 10.J. Godin, V. Lien, and Y. Lo, “Integrated fluidic photonics for multi-parameter in-plane detection in microfluidic flow cytometry,” in 19th Annual Meeting of the IEEE Lasers and Electro-Optics Society (Montreal, Quebec, 2006), pp. 605–606. [Google Scholar]
- 11.Lien V., Berdichevsky Y., Lo Y., “A prealigned process of integrating optical waveguides with microfluidic devices,” IEEE Photon. Technol. Lett. 16(6), 1525–1527 (2004). 10.1109/LPT.2004.826774 [DOI] [Google Scholar]
- 12.A. Yen, Flow Cytometry: Advanced Research and Clinical Applications, 7–8 (CRC Press, 1989).
- 13.Duffy D., McDonald J., Schueller O., Whitesides G. M., “Rapid prototyping of microfluidic systems in poly (dimethylsiloxane),” Anal. Chem. 70(23), 4974–4984 (1998). 10.1021/ac980656z [DOI] [PubMed] [Google Scholar]
- 14.E. Mantz, “MFI Homepage,” http://www.umass.edu/microbio/mfi/
- 15.Steen H. B., “Light scattering measurement in an arc lamp-based flow cytometer,” Cytometry 11(2), 223–230 (1990). 10.1002/cyto.990110202 [DOI] [PubMed] [Google Scholar]
- 16.S. Cho, J. Godin, C. Chen, F. Tsai, and Y. Lo, “Microfluidic photonic integrated circuits,” Proc. SPIE 7135, 713501 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen C. H., Cho S. H., Tsai F., Erten A., Lo Y. H., “Microfluidic cell sorter with integrated piezoelectric actuator,” Biomed. Microdevices 11(6), 1223–1231 (2009). 10.1007/s10544-009-9341-5 [DOI] [PMC free article] [PubMed] [Google Scholar]


