Figure 3.
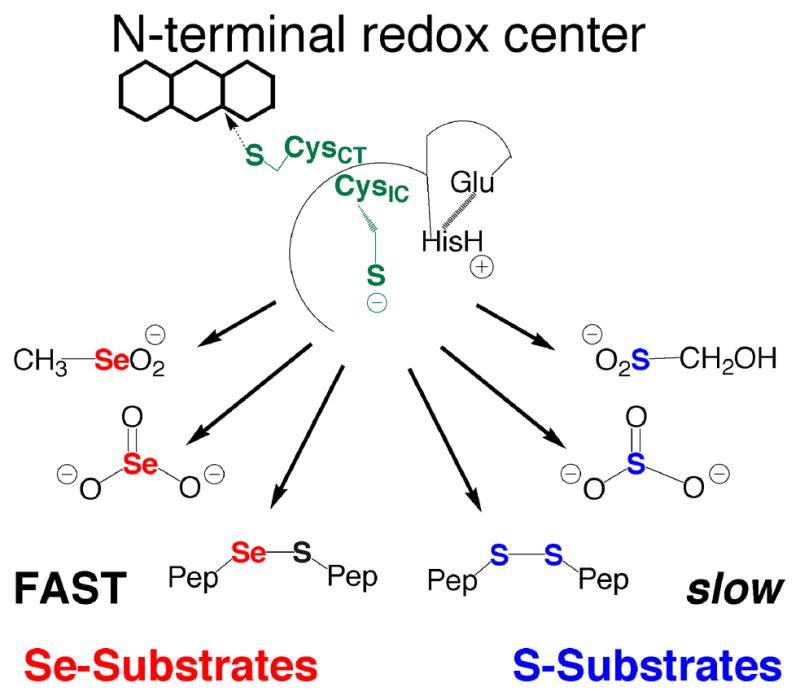
The importance of Se to substrate utilization by the N-terminal redox center. The N-terminal redox center will reduce small molecule Se-containing substrates such as SeO3−2, CH3SeO2−, and selenosulfide (Se–S) containing peptides. The S-analogs of these substrates are either not reduced at all such as HOCH2SO2− (reported here) and SO3−2 (10), or are reduced very slowly as in the case of cystine and other disulfide (S–S) containing peptides (12). We believe that the most likely reason that these Se-containing compounds are good substrates (reduced quickly) compared to the S-analogs is due to selenium’s strong ability to accept electrons (high electrophilicity). The explanation that high electrophilicity is the determining factor in substrate utilization by the N-terminal redox center is consistent with the fact that DTNB, lipoic acid, and quinones, all highly electrophilic compounds, are turned over by the N-terminal redox center as reported by us and others (20 and references therein).
