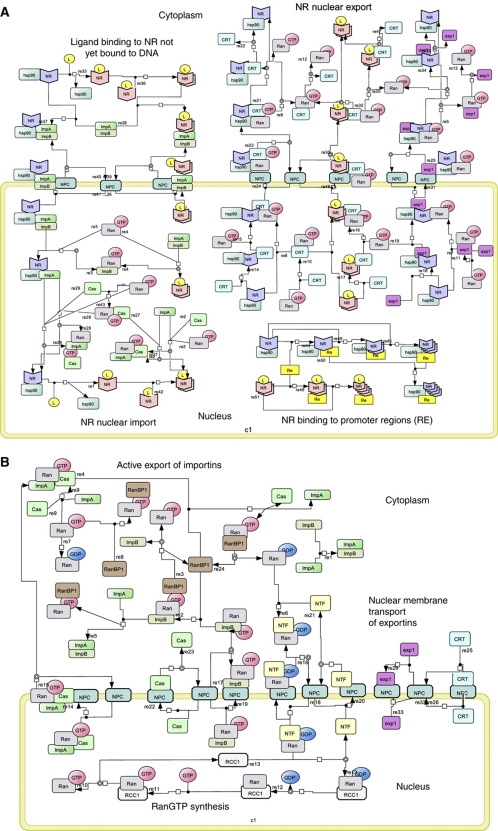
Figure 1.
(A, B) Network diagram for GR signaling. The NR signaling network is shown in terms of seven modules, in standard SBGN (Kitano et al, 2005). (A) (i) Ligand binding to NR not yet bound to DNA. Core-NR (indicated by the blue wedge shape) has its NLS1 masked by Hsp90, while its NLS2 is accessible. Both its NES1 and its NES2 are exposed. In this state the affinity of NR for DNA is low. Upon binding its ligand, the conformation of the NR is changed, the NR dimerizes, the NLS1 becomes unmasked, the NES2 is masked and the affinity of the NR to DNA is thereby increased. The consequent binding to the DNA (and the engagement of NR in active import into the nucleus, see below) shifts NR from cytoplasm to nucleus when ligand is added (Drouin et al, 1992). (ii) Reversible NR binding to REs: both liganded and core-NRs bind to REs and form tetramers. The DNA binding affinity for NRL is higher than that for core-NR (Garlatti et al, 1994). (iii) NR nuclear import: Both core and NRL bind to importin-α. Core-NR binds to importin-α due to the NLS2, but the NLS1 is occluded by Hsp90 protein. If the NR is liganded, both its NLS1 and its NLS2 are available. This provides higher affinity to importin-α (Pemberton and Paschal, 2005). Binding of the NR alters the conformation of importin-α such that its N terminus becomes accessible to importin-β, which, in turn, can interact with the nucleoporins in the NPC. The NPC allows the importins–cargo complex to pass the nuclear envelope (Sharova, 2002; Tran and Wente, 2006) The importin-β–importin-α–cargo complex binds RanGTP, which is exclusive to the nuclear compartment. Importin-β–importin-α–cargo–RanGTP complex dissociates into an importin-α–cargo and an importin-β–RanGTP moiety. Importin-α–cargo complex associates with RanGTP and CAS, which allows the cargo NR plus hormone to dissociate from the complex. RanGTP favors dissociation of the complexes and hence pushes the balance to dissociation of the cargo complexes in the nucleus, where association may be favored in the absence of RanGTP (i.e., in the cytosol). (iv) NR nuclear export: NR binds to exportin1 (CRM1) via NES1 or to calrecetin (CRT) via NES2. Both exportins bind to RanGTP and the resulting cargo–exportins–RanGTP complex passes through the NPC to the cytoplasm, where free RanGTP is hydrolyzed to RanGDP by RanGAP with the assistance of RanBP1. The lower level of RanGTP in the cytosol, as compared with the nucleus, favors dissociation of the complex into cargo, exportins and RanGTP. (B) (v) Active export of importins: both importin-α–RanGTP–Cas and importin-β–RanGTP complexes can move between nucleus and cytoplasm via the NPC. GAP associates with the NPC on the cytoplasmic side of the nuclear membrane, provoking the hydrolysis of RanGTP to RanGDP in both the complexes (Pemberton and Paschal, 2005), which then dissociate. GTP hydrolysis is assisted by the RanBP1 protein and coupled to the dissociation of RanGDP molecules from importins. (vi) Nuclear membrane transport of exportins: Exp1, CRT and Cas diffuse across the nuclear membrane through the NPC (Pemberton and Paschal, 2005). (vii) RanGTP synthesis: RanGDP is returned into the nucleus in a complex with transport factor NTF2 (Poon and Jans, 2005). The pool of RanGTP is restored when nuclear GEF (containing RCC1 protein and associated with chromatin (Macara, 2001)) replaces GDP with GTP in the Ran molecule (Pemberton and Paschal, 2005). The function of GEF is to provide a 4-step reversible reaction: RCC1 binds RanGDP, GDP is released from the complex, GTP binds to Ran-RCC1 and finally RCC1 splits from Ran-GTP (Riddick and Macara, 2007).