Figure 1.
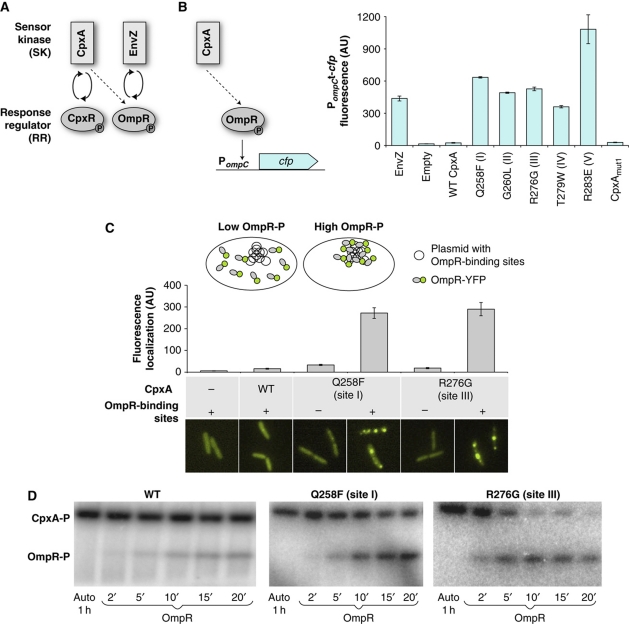
Amino-acid substitutions in CpxA identified in a scanning mutagenesis screen increase cross-talk to OmpR. (A) The SK/RR pairs EnvZ/OmpR and CpxA/CpxR. Weak cross-talk from CpxA to OmpR (dashed line), which is detectable in the absence of the cognate partners EnvZ and CpxR (Siryaporn and Goulian, 2008), is insensitive to signal input and lacks phosphatase activity. (B) Cross-talk to OmpR was measured from CFP fluorescence of an OmpR-regulated transcriptional reporter (ompC promoter (Siryaporn and Goulian, 2008)) in a strain that lacked EnvZ and CpxR, unless noted otherwise. Individual substitutions at sites I–V in CpxA (see Supplementary Figure S2) increase cross-talk to OmpR. The weak cross-talk from WT CpxA is too weak to be detectable in the scale of this figure. Reporter gene expression resulting from OmpR phosphorylation by EnvZ is shown for reference. Combining the substitutions at sites I–V (CpxAmut1) significantly lowers reporter gene expression. The bars denote averages and standard deviations of three independent experiments. Cultures of the ompC-cfp reporter strain AFS71 (envZ− cpxRA−) containing empty vector or plasmid that expresses EnvZ, wild-type CpxA, or CpxA mutants were grown in glycerol minimal medium. (C) Fluorescence localization assay for OmpR-YFP phosphorylation (Batchelor and Goulian, 2006; Siryaporn and Goulian, 2008). Conditions associated with high OmpR phosphorylation result in the formation of intense spots of OmpR-YFP fluorescence that co-localize with plasmid clusters, indicating increased DNA binding. Fluorescence localization in each cell was quantified by integrating a Gaussian that was fitted to the pixel intensities in a neighborhood around the brightest pixel, as described in Siryaporn and Goulian (2008). The data are averages of ∼100 cells. Cultures of OmpR-YFP strains containing chromosomal integrations of empty vector (AFS218) or chromosomal integrations of plasmids that express wild-type CpxA (AFS196) or CpxA mutants (AFS240 and AFS232) and containing either an empty vector (pEB123) or a plasmid with OmpR-binding sites (pEB124) were grown in glycerol minimal medium. (D) Cytoplasmic fragments of CpxA mutants containing individual substitutions show increased phosphotransfer to OmpR in vitro (see also Supplementary Figure S5). The first lane shows histidine-kinase autophosphorylation. The other lanes show phosphotransfer to OmpR at the indicated times (in minutes). See Supplementary information for a detailed description of the strains and growth conditions.